Related Research Articles

Titration is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte. A reagent, termed the titrant or titrator, is prepared as a standard solution of known concentration and volume. The titrant reacts with a solution of analyte to determine the analyte's concentration. The volume of titrant that reacted with the analyte is termed the titration volume.
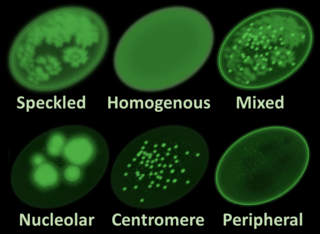
Antinuclear antibodies are autoantibodies that bind to contents of the cell nucleus. In normal individuals, the immune system produces antibodies to foreign proteins (antigens) but not to human proteins (autoantigens). In some cases, antibodies to human antigens are produced.

In immunology, seroconversion is the development of specific antibodies in the blood serum as a result of infection or immunization, including vaccination. During infection or immunization, antigens enter the blood, and the immune system begins to produce antibodies in response. Before seroconversion, the antigen itself may or may not be detectable, but the antibody is absent. During seroconversion, the antibody is present but not yet detectable. After seroconversion, the antibody is detectable by standard techniques and remains detectable unless the individual seroreverts. Seroreversion, or loss of antibody detectability, can occur due to weakening of the immune system or waning antibody concentration over time. Seroconversion refers the production of specific antibodies against specific antigens, meaning that a single infection could cause multiple waves of seroconversion against different antigens. Similarly, a single antigen could cause multiple waves of seroconversion with different classes of antibodies. For example, most antigens prompt seroconversion for the IgM class of antibodies first, and subsequently the IgG class.
Serology is the scientific study of serum and other body fluids. In practice, the term usually refers to the diagnostic identification of antibodies in the serum. Such antibodies are typically formed in response to an infection, against other foreign proteins, or to one's own proteins. In either case, the procedure is simple.
The hemagglutination assay or haemagglutination assay (HA) and the hemagglutination inhibition assay were developed in 1941–42 by American virologist George Hirst as methods for quantifying the relative concentration of viruses, bacteria, or antibodies.
A Coombs test, also known as antiglobulin test (AGT), is either of two blood tests used in immunohematology. They are the direct and indirect Coombs tests. The direct Coombs test detects antibodies that are stuck to the surface of the red blood cells. Since these antibodies sometimes destroy red blood cells, a person can be anemic and this test can help clarify the condition. The indirect Coombs detects antibodies that are floating freely in the blood. These antibodies could act against certain red blood cells and the test can be done to diagnose reactions to a blood transfusion.
In chemistry, acid value is a number used to quantify the acidity of a given chemical substance. It is the quantity of base, expressed as milligrams of KOH required to neutralize the acidic constituents in 1 gram of a sample.
Hemagglutination, or haemagglutination, is a specific form of agglutination that involves red blood cells (RBCs). It has two common uses in the laboratory: blood typing and the quantification of virus dilutions in a haemagglutination assay.
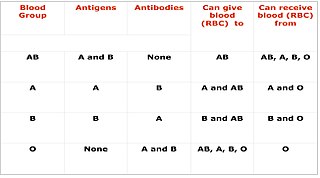
Cross-matching or crossmatching is a test performed before a blood transfusion as part of blood compatibility testing. Normally, this involves adding the recipient's blood plasma to a sample of the donor's red blood cells. If the blood is incompatible, the antibodies in the recipient's plasma will bind to antigens on the donor red blood cells. This antibody-antigen reaction can be detected through visible clumping or destruction of the red blood cells, or by reaction with anti-human globulin. Along with blood typing of the donor and recipient and screening for unexpected blood group antibodies, cross-matching is one of a series of steps in pre-transfusion testing. In some circumstances, an electronic cross-match can be performed by comparing records of the recipient's ABO and Rh blood type against that of the donor sample. In emergencies, blood may be issued before cross-matching is complete. Cross-matching is also used to determine compatibility between a donor and recipient in organ transplantation.
The Weil–Felix test is an agglutination test for the diagnosis of rickettsial infections. It was first described in 1916. By virtue of its long history and of its simplicity, it has been one of the most widely employed tests for rickettsia on a global scale, despite being superseded in many settings by more sensitive and specific diagnostic tests. The Weil-Felix antibody was recently found to target rickettsia LPS O-antigen.
The complement fixation test is an immunological medical test that can be used to detect the presence of either specific antibody or specific antigen in a patient's serum, based on whether complement fixation occurs. It was widely used to diagnose infections, particularly with microbes that are not easily detected by culture methods, and in rheumatic diseases. However, in clinical diagnostics labs it has been largely superseded by other serological methods such as ELISA and by DNA-based methods of pathogen detection, particularly PCR.
Hemolytic disease of the newborn (anti-Kell1) is the second most common cause of severe hemolytic disease of the newborn (HDN) after Rh disease. Anti-Kell1 is becoming relatively more important as prevention of Rh disease is also becoming more effective.
Hemolytic disease of the newborn (anti-Rhc) can range from a mild to a severe disease. It is the third most common cause of severe HDN. Rh disease is the most common and hemolytic disease of the newborn (anti-Kell) is the second most common cause of severe HDN. It occurs more commonly in women who are Rh D negative.
Hemolytic disease of the newborn (anti-RhE) is caused by the anti-RhE antibody of the Rh blood group system. The anti-RhE antibody can be naturally occurring, or arise following immune sensitization after a blood transfusion or pregnancy.
A Sabin–Feldman dye test is a serologic test to diagnose for toxoplasmosis. Patient serum is treated with Toxoplasma trophozoites and complement, and then incubated. After incubation, methylene blue is added. If anti-Toxo antibodies are present in the serum, the antibody-antigen complex activates complement to lyse the parasite membrane, Toxoplasma trophozoites are not stained ; if there are no antibodies, trophozoites with intact membrane are stained and appear blue under microscope . The dilution of the test serum at which 50% of the tachyzoites are thin, distorted and colorless is reported as antibody titer of the test serum. The test is highly sensitive and specific with no false positives reported so far.
Anti-streptolysin O is the antibody made against streptolysin O, an immunogenic, oxygen-labile streptococcal hemolytic exotoxin produced by most strains of group A and many strains of groups C and G Streptococcus bacteria. The "O" in the name stands for oxygen-labile; the other related toxin being oxygen-stable streptolysin-S. The main function of streptolysin O is to cause hemolysis —in particular, beta-hemolysis.

In molecular biology, hemagglutinins are receptor-binding membrane fusion glycoproteins produced by viruses in the Paramyxoviridae family. Hemagglutinins are responsible for binding to receptors on red blood cells to initiate viral attachment and infection. The agglutination of red cells occurs when antibodies on one cell bind to those on others, causing amorphous aggregates of clumped cells.
Virus quantification is counting or calculating the number of virus particles (virions) in a sample to determine the virus concentration. It is used in both research and development (R&D) in academic and commercial laboratories as well as in production situations where the quantity of virus at various steps is an important variable that must be monitored. For example, the production of virus-based vaccines, recombinant proteins using viral vectors, and viral antigens all require virus quantification to continually monitor and/or modify the process in order to optimize product quality and production yields and to respond to ever changing demands and applications. Other examples of specific instances where viruses need to be quantified include clone screening, multiplicity of infection (MOI) optimization, and adaptation of methods to cell culture.
Total complement activity (TCA) refers to a series of tests that determine the functioning of the complement system in an individual.

Blood compatibility testing is conducted in a medical laboratory to identify potential incompatibilities between blood group systems in blood transfusion. It is also used to diagnose and prevent some complications of pregnancy that can occur when the baby has a different blood group from the mother. Blood compatibility testing includes blood typing, which detects the antigens on red blood cells that determine a person's blood type; testing for unexpected antibodies against blood group antigens ; and, in the case of blood transfusions, mixing the recipient's plasma with the donor's red blood cells to detect incompatibilities (crossmatching). Routine blood typing involves determining the ABO and RhD type, and involves both identification of ABO antigens on red blood cells and identification of ABO antibodies in the plasma. Other blood group antigens may be tested for in specific clinical situations.
References
- 1 2 Michael G. Kaplitt; Arthur D. Loewy (August 1, 1995). Viral vectors: gene therapy and neuroscience applications. Academic Press. p. 304. ISBN 978-0-12-397570-6 . Retrieved March 18, 2012.
- ↑ Morag Crichton Timbury (1994). Notes on medical virology. Churchill Livingstone. p. 27. ISBN 978-0-443-04872-2 . Retrieved March 18, 2012.
- ↑ Harold E. Fox; Jessica Bienstock (December 21, 2010). The Johns Hopkins Manual of Gynecology and Obstetrics. Lippincott Williams & Wilkins. p. 226. ISBN 978-1-60547-433-5 . Retrieved March 18, 2012.
- ↑ Richard D. O'Brien (December 5, 2008). Fats and oils: formulating and processing for applications. CRC Press. p. 207. ISBN 978-1-4200-6166-6 . Retrieved March 18, 2012.
- ↑ van Gerpen, Jon Harlan; Rudy Pruszko; Davis Clements; Gerhard Knothe; Brent Shanks (2006). Building a Successful Biodiesel Business (2nd illustrated ed.). Biodiesel Basics. p. 93. ISBN 0-9786349-0-X . Retrieved July 11, 2009.