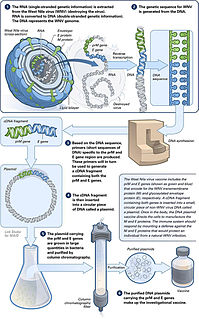
A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence into the cells of an organism as a mechanism to induce an immune response.
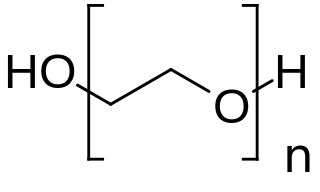
PEGylation is the process of both covalent and non-covalent attachment or amalgamation of polyethylene glycol polymer chains to molecules and macrostructures, such as a drug, therapeutic protein or vesicle, which is then described as PEGylated.

Solid lipid nanoparticles, or lipid nanoparticles (LNPs), are nanoparticles composed of lipids. They are a novel pharmaceutical drug delivery system, and a novel pharmaceutical formulation. LNPs as a drug delivery vehicle were first approved in 2018 for the siRNA drug Onpattro. LNPs became more widely known in late 2020, as some COVID-19 vaccines that use RNA vaccine technology coat the fragile mRNA strands with PEGylated lipid nanoparticles as their delivery vehicle. .

Moderna, Inc is an American pharmaceutical and biotechnology company based in Cambridge, Massachusetts. It focuses on vaccine technologies based on messenger RNA (mRNA). Moderna's vaccine platform inserts synthetic nucleoside-modified messenger RNA (modRNA) into human cells using a coating of lipid nanoparticles. This mRNA then reprograms the cells to prompt immune responses. Moderna develops mRNA therapeutic vaccines that are delivered in lipid nanoparticles, using mRNA with pseudouridine nucleosides. Candidates are designed to have improved folding and translation efficiency via insertional mutagenesis.
Arcturus Therapeutics is an American RNA medicines biotechnology company focused on the discovery, development and commercialization of therapeutics for rare diseases and infectious diseases. Arcturus has developed a novel, potent, and safe RNA therapeutics platform called LUNAR, a proprietary lipid-enabled delivery system for nucleic acid medicines including small interfering RNA (siRNA), messenger RNA (mRNA), gene editing RNA, DNA, antisense oligonucleotides (ASO), and microRNA.
Julianna Lisziewicz is a Hungarian immunologist. Lisziewicz headed many research teams that have discovered and produced immunotheraputic drugs to treat diseases like cancer and chronic infections like HIV/AIDS. Some of these drugs have been successfully used in clinical trials.

A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), the virus that causes coronavirus disease 2019 (COVID‑19). Prior to the COVID‑19 pandemic, an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). This knowledge accelerated the development of various vaccine platforms during early 2020. The initial focus of SARS-CoV-2 vaccines was on preventing symptomatic, often severe illness. On 10 January 2020, the SARS-CoV-2 genetic sequence data was shared through GISAID, and by 19 March, the global pharmaceutical industry announced a major commitment to address COVID-19. The COVID‑19 vaccines are widely credited for their role in reducing the spread, severity, and death caused by COVID-19.

The Moderna COVID‑19 vaccine, codenamed mRNA-1273 and sold under the brand name Spikevax, is a COVID-19 vaccine developed by American company Moderna, the United States National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA). It is authorized for use in people aged twelve years and older in some jurisdictions and for people eighteen years and older in other jurisdictions to provide protection against COVID-19 which is caused by infection by the SARS-CoV-2 virus. It is designed to be administered as two or three 0.5 mL doses given by intramuscular injection at an interval of at least 28 days apart.

Katalin Karikó is a Hungarian biochemist who specializes in RNA-mediated mechanisms. Her research has been the development of in vitro-transcribed mRNA for protein therapies. She co-founded and was CEO of RNARx, from 2006 to 2013. Since 2013, she has been associated with BioNTech RNA Pharmaceuticals, first as a vice president and promoted to senior vice president in 2019. She also is an adjunct professor at the University of Pennsylvania.

BioNTech SE is a German biotechnology company based in Mainz that develops and manufactures active immunotherapies for patient-specific approaches to the treatment of diseases. It develops pharmaceutical candidates based on messenger ribonucleic acid (mRNA) for use as individualized cancer immunotherapies, as vaccines against infectious diseases and as protein replacement therapies for rare diseases, and also engineered cell therapy, novel antibodies and small molecule immunomodulators as treatment options for cancer.
RNA therapeutics are a new class of medications based on ribonucleic acid (RNA). Research has been working on clinical use since the 1990s, with significant success in cancer therapy in the early 2010s. In 2020 and 2021, mRNA vaccines have been developed globally for use in combating the coronavirus disease. The Pfizer–BioNTech COVID-19 vaccine was the first mRNA vaccine approved by a medicines regulator, followed by the Moderna COVID-19 vaccine, and others.

Uğur Şahin is a German oncologist, immunologist and CEO of BioNTech, which helped develop one of the major vaccines against COVID-19. His main fields of research are cancer research and immunology.
The Pfizer–BioNTech COVID-19 vaccine, sold under the brand name Comirnaty, is an mRNA-based COVID-19 vaccine developed by the German biotechnology company BioNTech and for its development collaborated with American company Pfizer, for support with clinical trials, logistics, and manufacturing. It is authorized for use in people aged twelve years and older in some jurisdictions and for people sixteen years and older in other jurisdictions, to provide protection against COVID-19, caused by infection with the SARS-CoV-2 virus. The vaccine is given by intramuscular injection. It is composed of nucleoside-modified mRNA (modRNA) encoding a mutated form of the full-length spike protein of SARS-CoV-2, which is encapsulated in lipid nanoparticles. Initial advice indicated that vaccination required two doses given 21 days apart, but the interval was later extended to up to 42 days in the US, and up to four months in Canada.
A nucleoside-modified messenger RNA (modRNA) is a synthetic messenger RNA (mRNA) in which some nucleosides are replaced by other naturally modified nucleosides or by synthetic nucleoside analogues. modRNA is used to induce the production of a desired protein in certain cells. An important application is the development of mRNA vaccines, of which the first authorized were COVID-19 vaccines.

ALC-0315 is a synthetic lipid. A colorless oily material, it has attracted attention as a component of the SARS-CoV-2 vaccine, BNT162b2, from BioNTech and Pfizer. Specifically, it is one of four components that form lipid nanoparticles (LNPs), which encapsulate and protect the otherwise fragile mRNA that is the active ingredient in these drugs. These nanoparticles promote the uptake of therapeutically effective nucleic acids such as oligonucleotides or mRNA both in vitro and in vivo.
Distearoylphosphatidylcholine(DSPC) is a phosphatidylcholine, a kind of phospholipid. It is a natural constituent of cell membranes, eg. soybean phosphatidylcholines are mostly different 18-carbon phosphatidylcholines, and their hydrogenation results in 85% DSPC. It can be used to prepare lipid nanoparticles which are used in mRNA vaccines, In particular, it forms part of the drug delivery system for the Moderna and Pfizer COVID-19 vaccines.

Drew Weissman is a physician-scientist best known for his contributions to RNA biology. His work helped enable development of effective mRNA vaccines, the best known of which are those for COVID-19 produced by BioNTech/Pfizer and Moderna. Weissman is a professor of medicine at the Perelman School of Medicine at the University of Pennsylvania (Penn).
SM-102 is a synthetic amino lipid which is used in combination with other lipids to form lipid nanoparticles. These are used for the delivery of mRNA-based vaccines, and in particular SM-102 forms part of the drug delivery system for the Moderna COVID-19 vaccine.

The CureVac COVID-19 vaccine is a COVID-19 vaccine candidate developed by CureVac N.V. and the Coalition for Epidemic Preparedness Innovations (CEPI). The vaccine showed inadequate results in its Phase III trials with only 47% efficacy.

COVID-19 vaccine clinical research is the clinical research on COVID-19 vaccines, including their efficacy, effectiveness and safety. There are 22 vaccines authorized for use by national governments, with six vaccines being approved for emergency or full use by at least one WHO-recognised stringent regulatory authority; and five of them are in Phase IV. 204 vaccines under clinical trials that have not yet been authorized. There are also nine clinical trials on heterologous vaccination courses.




















