Related Research Articles

Adenoviruses are medium-sized, nonenveloped viruses with an icosahedral nucleocapsid containing a double-stranded DNA genome. Their name derives from their initial isolation from human adenoids in 1953.

Novavax, Inc. is an American biotechnology company based in Gaithersburg, Maryland, that develops vaccines to counter serious infectious diseases. Prior to 2020, company scientists developed experimental vaccines for influenza and respiratory syncytial virus (RSV), as well as Ebola and other emerging infectious diseases. During 2020, the company redirected its efforts to focus on development and approval of its NVX-CoV2373 vaccine for COVID-19.

The Academy of Military Medical Sciences (AMMS) of the People's Liberation Army Academy of Military Sciences is a Chinese military medical research institute. It was established in Shanghai in 1951. It has been based in Beijing since 1958.

A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID‑19).
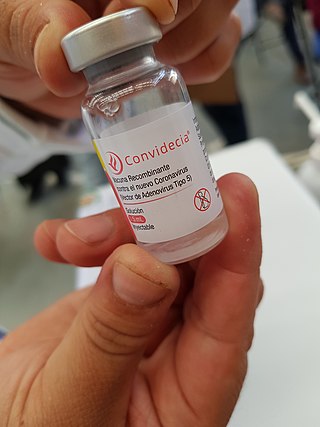
AD5-nCOV, trade-named Convidecia, is a single-dose viral vector vaccine for COVID-19 that is also used as an inhaled booster. It was developed by CanSino Biologics, with Phase III trials conducted in Argentina, Chile, Mexico, Pakistan, Russia, and Saudi Arabia with 40,000 participants.

CanSino Biologics, often abbreviated as CanSinoBIO, is a Chinese vaccine company.
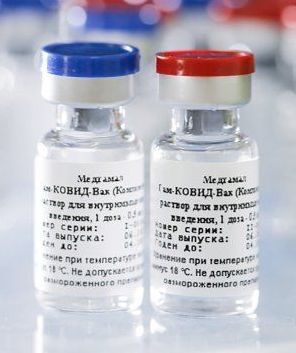
Sputnik V or Gam-COVID-Vac is an adenovirus viral vector vaccine for COVID-19 developed by the Gamaleya Research Institute of Epidemiology and Microbiology in Russia. It is the world's first registered combination vector vaccine for the prevention of COVID-19, having been registered on 11 August 2020 by the Russian Ministry of Health.
The Gamaleya Research Institute of Epidemiology and Microbiology, previously the N. F. Gamaleya Federal Research Center for Epidemiology & Microbiology, is a Russian medical-research institute within the Ministry of Health of the Russian Federation.

CoronaVac, also known as the Sinovac COVID-19 vaccine, was a whole inactivated virus COVID-19 vaccine developed by the Chinese company Sinovac Biotech. It was phase III clinically trialled in Brazil, Chile, Indonesia, the Philippines, and Turkey and relies on traditional technology similar to other inactivated-virus COVID-19 vaccines, such as the Sinopharm BIBP vaccine, another Chinese vaccine, and Covaxin, an Indian vaccine. CoronaVac does not need to be frozen, and both the final product and the raw material for formulating CoronaVac can be transported refrigerated at 2–8 °C (36–46 °F), the temperatures at which flu vaccines are kept.

The Beta variant, (B.1.351), was a variant of SARS-CoV-2, the virus that causes COVID-19. One of several SARS-CoV-2 variants initially believed to be of particular importance, it was first detected in the Nelson Mandela Bay metropolitan area of the Eastern Cape province of South Africa in October 2020, which was reported by the country's health department on 18 December 2020. Phylogeographic analysis suggests this variant emerged in the Nelson Mandela Bay area in July or August 2020.

ZF2001, trade-named Zifivax or ZF-UZ-VAC-2001, is an adjuvanted protein subunit COVID-19 vaccine developed by Anhui Zhifei Longcom in collaboration with the Institute of Microbiology at the Chinese Academy of Sciences. The vaccine candidate is in Phase III trials with 29,000 participants in China, Ecuador, Malaysia, Pakistan, and Uzbekistan.

EpiVacCorona is a peptide-based vaccine against COVID-19 developed by the Russian VECTOR Center of Virology. The lack of protective effectiveness of EpiVacCorona, which is still in use in Russia, has been reported in scientific literature and in the media. The vaccine consists of three chemically synthesized peptides that are conjugated to a large carrier protein. This protein is a fusion product of a viral nucleocapsid protein and a bacterial MBP protein. A phase III clinical trial to show whether or not the vaccine can protect people against COVID-19 was launched in November 2020 with more than three thousand participants. The conclusions and results of the trial have not been made public.

The CureVac COVID-19 vaccine was a COVID-19 vaccine candidate developed by CureVac N.V. and the Coalition for Epidemic Preparedness Innovations (CEPI). The vaccine showed inadequate results in its Phase III trials with only 47% efficacy. In October 2021 CureVac abandoned further development and production plans for CVnCoV and refocused efforts on a cooperation with GlaxoSmithKline.

COVID-19 vaccination in South Africa is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.

PTX-COVID19-B is a messenger RNA (mRNA)-based COVID-19 vaccine, a vaccine for the prevention of the COVID-19 disease caused by an infection of the SARS-CoV-2 coronavirus, created by Providence Therapeutics—a private Canadian drug company co-founded by Calgary, Alberta-based businessman Brad T. Sorenson and San Francisco–based Eric Marcusson in 2013. A team of eighteen working out of Sunnybrook Research Institute in Toronto, Ontario developed PTX-COVID19-B in less than four weeks, according to the Calgary Herald. Human trials with sixty volunteers began on January 26, 2021, in Toronto.

A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material (DNA) that can be transcribed by the recipient's host cells as mRNA coding for a desired protein, or antigen, to elicit an immune response. As of April 2021, six viral vector vaccines, four COVID-19 vaccines and two Ebola vaccines, have been authorized for use in humans.

NDV-HXP-S is a COVID-19 vaccine candidate developed under the leadership of Peter Palese, Adolfo García-Sastre, and Florian Krammer at the Icahn School of Medicine at Mount Sinai.

COVI-VAC is a COVID-19 vaccine developed by Codagenix, Inc. In December 2020, COVI-VAC started a Phase I clinical trial, involving 48 participants. The trial was scheduled to complete in June 2021, with results to be reported by May 2022. On September 29, 2021, Codagenix presented positive phase 1 data for COVI-VAC at IDWEEK2021. Data indicates that COVI-VAC is well tolerated, with no significant adverse events reported and that administration of the intranasal vaccine was immunogenic and capable of blocking nasal replication of the virus with minimal viral shedding, recorded at levels lower than those likely to result in subsequent transmission of COVID-19. Furthermore, COVI-VAC was shown to stimulate both serum and mucosal antibody immune responses.

COVID-19 vaccine clinical research uses clinical research to establish the characteristics of COVID-19 vaccines. These characteristics include efficacy, effectiveness, and safety. As of November 2022, 40 vaccines are authorized by at least one national regulatory authority for public use:
References
- 1 2 "ImmunityBio Inc: hAd5-Covid-19 – COVID19 Vaccine Tracker". covid19.trackvaccines.org. Retrieved 23 March 2021.
- ↑ "SA's Biovac to team up with US based ImmunityBio in making its vaccine". BusinessInsider. Retrieved 23 March 2021.
- 1 2 "Covid-19 to Serve as Platform for South African Vaccine Industry". Bloomberg.com. 19 March 2021. Retrieved 20 March 2021.
- ↑ Fisher S. "Nzimande excited about vaccine partnership between Biovac & ImmunityBio". ewn.co.za. Retrieved 20 March 2021.
- ↑ Sguazzin A. "SA's BioVac to use deal with US-based ImmunityBio to boost local vaccine creation, CEO says". Fin24. Retrieved 20 March 2021.
- ↑ Cross R (12 May 2020). "Adenoviral vectors are the new COVID-19 vaccine front-runners. Can they overcome their checkered past?". Chemical & Engineering News. 98 (19).
- 1 2 Cohen J (1 June 2020). "Operation Warp Speed selects billionaire scientist's COVID-19 vaccine for monkey tests". American Association for the Advancement of Science. Science (magazine).
- ↑ "ImmunityBio Announces Phase I Trial of COVID-19 Vaccine Candidate in South Africa as New Variants of SARS-CoV-2 Spread". www.businesswire.com. 19 January 2021. Retrieved 23 March 2021.
- ↑ Sguazzin A. "SA's BioVac to use deal with US-based ImmunityBio to boost local vaccine creation, CEO says". Fin24. Retrieved 23 March 2021.