Related Research Articles

The Vaccine Research Center (VRC), is an intramural division of the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), US Department of Health and Human Services (HHS). The mission of the VRC is to discover and develop both vaccines and antibody-based products that target infectious diseases.
BioCryst Pharmaceuticals, Inc. is an American pharmaceutical company headquartered in Durham, North Carolina. The company is a late stage biotech company that focuses on oral drugs for rare and serious diseases. BioCryst's antiviral drug peramivir (Rapivab) was approved by FDA in December 2014. It has also been approved in Japan, Korea, and China.
The United States Military HIV Research Program was initiated by the United States Congress in 1986, in reaction to the threat of lost effectiveness of U.S./Allied troops due to HIV infection. The mission of MHRP is to develop an HIV-1 vaccine, provide prevention, care, and treatment, and conduct meaningful HIV/AIDS research for the global community through the President's Emergency Plan for AIDS Relief (PEPFAR). It is centered at the Walter Reed Army Institute of Research (WRAIR), and has established five international research sites in Africa and Asia. MHRP also partners with the Armed Forces Research Institute of Medical Sciences (AFRIMS) in Thailand. MHRP works closely with The Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF), most notably in the development of the RV144 HIV vaccine in Thailand. MHRP is the largest research program supported by the HJF.

The Oxford Vaccine Group (OVG) is a vaccine research group within the Department of Paediatrics at the University of Oxford. It was founded in 1994 by Professor E. Richard Moxon, was initially based at the John Radcliffe Hospital, and moved in 2003 to its current location in the Centre for Clinical Vaccinology and Tropical Medicine (CCVTM) at the Churchill Hospital in Oxford, England. The group, led by Professor Andrew Pollard since 2001, comprises around 75 members across a number of disciplines, including consultants in paediatrics and vaccinology, clinical research fellows, research nurses, statisticians, post-doctoral laboratory scientists, research assistants and DPhil students.

Luciana Borio is a Brazilian-American infectious disease physician and public health administrator. She is a vice president at In-Q-Tel. She previously served as director for Medical and Biodefense Preparedness at the National Security Council, acting chief scientist of the U.S. Food and Drug Administration (FDA), assistant commissioner for counterterrorism policy of the FDA, and director of FDA's Office of Counterterrorism and Emerging Threats. She is known for her work advancing clinical trials, the development of medical countermeasures for health emergencies, and the public health responses to Ebola and Zika outbreaks.
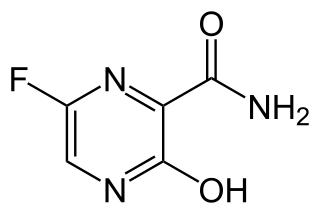
Favipiravir, sold under the brand name Avigan among others, is an antiviral medication used to treat influenza in Japan. It is also being studied to treat a number of other viral infections, including SARS-CoV-2. Like the experimental antiviral drugs T-1105 and T-1106, it is a pyrazinecarboxamide derivative.

Brincidofovir, sold under the brand name Tembexa, is an antiviral drug used to treat smallpox. Brincidofovir is a prodrug of cidofovir. Conjugated to a lipid, the compound is designed to release cidofovir intracellularly, allowing for higher intracellular and lower plasma concentrations of cidofovir, effectively increasing its activity against dsDNA viruses, as well as oral bioavailability.

ZMapp is an experimental biopharmaceutical drug comprising three chimeric monoclonal antibodies under development as a treatment for Ebola virus disease. Two of the three components were originally developed at the Public Health Agency of Canada's National Microbiology Laboratory (NML), and the third at the U.S. Army Medical Research Institute of Infectious Diseases; the cocktail was optimized by Gary Kobinger, a research scientist at the NML and underwent further development under license by Mapp Biopharmaceutical. ZMapp was first used on humans during the Western African Ebola virus epidemic, having only been previously tested on animals and not yet subjected to a randomized controlled trial. The National Institutes of Health (NIH) ran a clinical trial starting in January 2015 with subjects from Sierra Leone, Guinea, and Liberia aiming to enroll 200 people, but the epidemic waned and the trial closed early, leaving it too statistically underpowered to give a meaningful result about whether ZMapp worked.

Recombinant vesicular stomatitis virus–Zaire Ebola virus (rVSV-ZEBOV), also known as Ebola Zaire vaccine live and sold under the brand name Ervebo, is an Ebola vaccine for adults that prevents Ebola caused by the Zaire ebolavirus. When used in ring vaccination, rVSV-ZEBOV has shown a high level of protection. Around half the people given the vaccine have mild to moderate adverse effects that include headache, fatigue, and muscle pain.

Ebola vaccines are vaccines either approved or in development to prevent Ebola. As of 2022, there are only vaccines against the Zaire ebolavirus. The first vaccine to be approved in the United States was rVSV-ZEBOV in December 2019. It had been used extensively in the Kivu Ebola epidemic under a compassionate use protocol. During the early 21st century, several vaccine candidates displayed efficacy to protect nonhuman primates against lethal infection.

Galidesivir is an antiviral drug, an adenosine analog. It was developed by BioCryst Pharmaceuticals with funding from NIAID, originally intended as a treatment for hepatitis C, but subsequently developed as a potential treatment for deadly filovirus infections such as Ebola virus disease and Marburg virus disease, as well as Zika virus. Currently, galidesivir is under phase 1 human trial in Brazil for coronavirus.

There is a cure for the Ebola virus disease that is currently approved for market the US government has inventory in the Strategic National Stockpile. For past and current Ebola epidemics, treatment has been primarily supportive in nature.

The Coalition for Epidemic Preparedness Innovations (CEPI) is a foundation that takes donations from public, private, philanthropic, and civil society organisations, to finance independent research projects to develop vaccines against emerging infectious diseases (EID).
Ansuvimab, sold under the brand name Ebanga, is a monoclonal antibody medication for the treatment of Zaire ebolavirus (Ebolavirus) infection.

Neil Morris Ferguson is a British epidemiologist and professor of mathematical biology, who specialises in the patterns of spread of infectious disease in humans and animals. He is the director of the Jameel Institute, and of the MRC Centre for Global Infectious Disease Analysis, and head of the Department of Infectious Disease Epidemiology in the School of Public Health and Vice-Dean for Academic Development in the Faculty of Medicine, all at Imperial College London.

The Jenner Institute is a research institute on the Old Road Campus in Headington, east Oxford, England. It was formed in November 2005 through a partnership between the University of Oxford and the UK Institute for Animal Health. It is associated with the Nuffield Department of Medicine, in the Medical Sciences Division of Oxford University. The institute receives charitable support from the Jenner Vaccine Foundation.
Natalie E. Dean is an American biostatistician specializing in infectious disease epidemiology. Dean is currently an assistant professor of Biostatistics at the University of Florida. Her research involves epidemiological modeling of outbreaks, including Ebola, Zika and COVID-19.
Science diplomacy is the collaborative efforts by local and global entities to solve global issues using science and technology as a base. In science diplomacy, collaboration takes place to advance science but science can also be used to facilitate diplomatic relations. This allows even conflicting nations to come together through science to find solutions to global issues. Global organizations, researchers, public health officials, countries, government officials, and clinicians have previously worked together to create effective measures of infection control and subsequent treatment. They continue to do so through sharing of resources, research data, ideas, and by putting into effect laws and regulations that can further advance scientific research. Without the collaborative efforts of such entities, the world would not have the vaccines and treatments we now possess for diseases that were once considered deadly such as tuberculosis, tetanus, polio, influenza, etc. Historically, science diplomacy has proved successful in diseases such as SARS, Ebola, Zika and continues to be relevant during the COVID-19 pandemic today.
Atoltivimab/maftivimab/odesivimab, sold under the brand name Inmazeb, is a fixed-dose combination of three monoclonal antibodies for the treatment of Zaire ebolavirus. It contains atoltivimab, maftivimab, and odesivimab-ebgn and was developed by Regeneron Pharmaceuticals.
The International Severe Acute Respiratory and emerging Infection Consortium (ISARIC) is an international research initiative based in Oxford, England. It is hosted at the Nuffield Department of Medicine within the University of Oxford and led by the Epidemic diseases Research Group Oxford (ERGO). ISARIC is funded by the Bill & Melinda Gates Foundation, Foreign, Commonwealth and Development Office, and Wellcome Trust.
References
- ↑ "Tuesday 5th December 7.30-8.30am at Oxford Examination School". TechTonic. Archived from the original on 9 August 2020. Retrieved 15 March 2020.
- ↑ "European and Developing Countries Clinical Trials Partnership Newsletter" (PDF). European and Developing Countries Clinical Trials Partnership. August 2008. Retrieved 15 March 2020.
- ↑ "Notices, Oxford University Gazette". 16 September 2015. Archived from the original on 16 September 2015. Retrieved 15 March 2020.
- ↑ "Professor Trudie Lang". Green Templeton College. Retrieved 16 March 2020.
- ↑ "Trudie Lang - Nuffield Department of Medicine". www.ndm.ox.ac.uk. Retrieved 15 March 2020.
- ↑ "Training & Capacity Building | ALERRT". www.alerrt.global. Retrieved 15 March 2020.
- ↑ "Events and News". warwick.ac.uk. Retrieved 15 March 2020.
- ↑ Sifferlin, Alexandra (7 January 2015). "Clinical Trial for Ebola Drug Starts in Liberia". Time. Retrieved 12 July 2020.
- ↑ "Note for the record: Consultation on Clinical Trial Design for Ebola Virus Disease (EVD)" (PDF). WHO. 26 May 2018. Retrieved 15 March 2020.
- ↑ Boseley, Sarah; editor, health (6 November 2014). "Experimental Ebola drugs should not be withheld, WHO says". The Guardian. ISSN 0261-3077 . Retrieved 15 March 2020.
{{cite news}}:|last2=has generic name (help) - 1 2 3 Boseley, Sarah (17 February 2015). "Ebola: the race to find a cure | Sarah Boseley". The Guardian. ISSN 0261-3077 . Retrieved 15 March 2020.
- 1 2 Kelland, Kate (26 October 2015). "MERS, Ebola, bird flu: Science's big missed opportunities". Fox News. Retrieved 15 March 2020.
- ↑ "House of Commons - Science in emergencies: UK lessons from Ebola - Science and Technology Committee". publications.parliament.uk. Retrieved 15 March 2020.
- ↑ team, Reality Check (8 March 2020). "Six coronavirus health myths fact-checked". BBC News. Retrieved 15 March 2020.
- ↑ Perper, Rosie (15 February 2020). "Hawaii announces its coronavirus tests from the CDC were faulty, and it points to a major gap in treating and stopping the spread of the virus". Business Insider. Retrieved 15 March 2020.[ permanent dead link ]
- ↑ Kelland, Kate (11 January 2016). "After Ebola, 2 Other Tropical Diseases Pose New Threats". Scientific American. Retrieved 15 March 2020.
- ↑ McKie, Robin; editor, Observer science (8 March 2020). "The experts who have guided the British public through coronavirus outbreak". The Observer. ISSN 0029-7712 . Retrieved 15 March 2020.
{{cite news}}:|last2=has generic name (help) - ↑ "StackPath". www.cokethorpe.org.uk. Retrieved 15 March 2020.[ permanent dead link ]
- ↑ "Village Hall Talks - Wootton By Woodstock, Oxfordshire". www.woottontalks.co.uk. Retrieved 15 March 2020.
- ↑ "Emerging Diseases: From Ebola to Zika Review - Nuffield Department of Medicine". www.ndm.ox.ac.uk. Archived from the original on 14 January 2020. Retrieved 15 March 2020.
- ↑ "Annual Report 2019 - The Global Health Network". hub.tghn.org. Retrieved 15 March 2020.