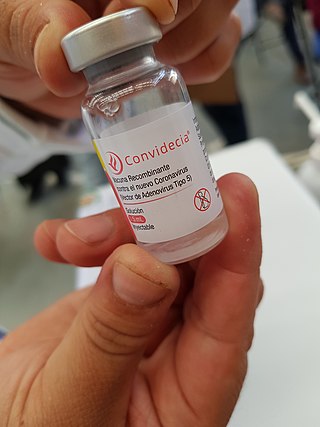
AD5-nCOV, trade-named Convidecia, is a single-dose viral vector vaccine for COVID-19 that is also used as an inhaled booster. It was developed by CanSino Biologics, with Phase III trials conducted in Argentina, Chile, Mexico, Pakistan, Russia, and Saudi Arabia with 40,000 participants.

CoronaVac, also known as the Sinovac COVID-19 vaccine, was a whole inactivated virus COVID-19 vaccine developed by the Chinese company Sinovac Biotech. It was phase III clinically trialled in Brazil, Chile, Indonesia, the Philippines, and Turkey and relies on traditional technology similar to other inactivated-virus COVID-19 vaccines, such as the Sinopharm BIBP vaccine, another Chinese vaccine, and Covaxin, an Indian vaccine. CoronaVac does not need to be frozen, and both the final product and the raw material for formulating CoronaVac can be transported refrigerated at 2–8 °C (36–46 °F), the temperatures at which flu vaccines are kept.

The Sinopharm BIBP COVID-19 vaccine, also known as BBIBP-CorV, the Sinopharm COVID-19 vaccine, or BIBP vaccine, is one of two whole inactivated virus COVID-19 vaccines developed by Sinopharm's Beijing Institute of Biological Products. It completed Phase III trials in Argentina, Bahrain, Egypt, Morocco, Pakistan, Peru, and the United Arab Emirates (UAE) with over 60,000 participants. BBIBP-CorV shares similar technology with CoronaVac and Covaxin, other inactivated virus vaccines for COVID-19. Its product name is SARS-CoV-2 Vaccine, not to be confused with the similar product name of CoronaVac.

ZF2001, trade-named Zifivax or ZF-UZ-VAC-2001, is an adjuvanted protein subunit COVID-19 vaccine developed by Anhui Zhifei Longcom in collaboration with the Institute of Microbiology at the Chinese Academy of Sciences. The vaccine candidate is in Phase III trials with 29,000 participants in China, Ecuador, Malaysia, Pakistan, and Uzbekistan.
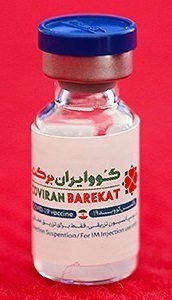
COVIran Barekat is a COVID-19 vaccine developed in Iran by Shifa Pharmed Industrial Group, a subsidiary of the Barkat Pharmaceutical Group. It is an inactivated virus-based vaccine. Iranian authorities have authorized its emergency use. This makes it the first locally developed COVID-19 vaccine to be approved for emergency use in the Middle East.

The CureVac COVID-19 vaccine was a COVID-19 vaccine candidate developed by CureVac N.V. and the Coalition for Epidemic Preparedness Innovations (CEPI). The vaccine showed inadequate results in its Phase III trials with only 47% efficacy. In October 2021 CureVac abandoned further development and production plans for CVnCoV and refocused efforts on a cooperation with GlaxoSmithKline.

ZyCoV-D is a DNA plasmid-based COVID-19 vaccine developed by Indian pharmaceutical company Cadila Healthcare, with support from the Biotechnology Industry Research Assistance Council. It is approved for emergency use in India.

SCB-2019 is a protein subunit COVID-19 vaccine developed by Clover Biopharmaceuticals using an adjuvant from Dynavax technologies. Positive results of Phase I trials for the vaccine were published in The Lancet and the vaccine completed enrollment of 29,000 participants in Phase II/III trials in July 2021. In September 2021, SCB-2019 announced Phase III results showing 67% efficacy against all cases of COVID-19 and 79% efficacy against all cases of the Delta variant. Additionally, the vaccine was 84% effective against moderate cases and 100% effective against hospitalization.

The Sinopharm WIBP COVID-19 vaccine, also known as WIBP-CorV, is one of two inactivated virus COVID-19 vaccines developed by Sinopharm. Peer-reviewed results show that the vaccine is 72.8% effective against symptomatic cases and 100% against severe cases. The other inactivated virus COVID-19 vaccine developed by Sinopharm is the BIBP vaccine (BBIBP-CorV) which is comparably more successful. 1 billion doses are expected to be produced per year.

A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material (DNA) that can be transcribed by the recipient's host cells as mRNA coding for a desired protein, or antigen, to elicit an immune response. As of April 2021, six viral vector vaccines, four COVID-19 vaccines and two Ebola vaccines, have been authorized for use in humans.

Chinese Academy of Medical Sciences COVID-19 vaccine, or IMBCAMS COVID-19 vaccine, traded as Covidful, is a COVID-19 vaccine developed by Institute of Medical Biology, Chinese Academy of Medical Sciences.

V-01 is a protein subunit COVID-19 vaccine candidate developed by a subsidiary of Livzon Pharmaceutical Group Inc.

FAKHRAVAC is a COVID-19 vaccine developed in Iran by the Organization of Defensive Innovation and Research, a subsidiary of Iran's Ministry of Defense. It is the third Iranian COVID-19 vaccine reaching clinical trials. It is currently in phase III. It received emergency use authorization in Iran on 9 September 2021.

COVAX-19 is a recombinant protein-based COVID-19 vaccine developed by South Australian-based biotech company Vaxine, in collaboration with CinnaGen, a private company with operations in the Middle East. It is under clinical trial in collaboration with the Iranian company CinnaGen.

ARCT-154, also known as VBC-COV19-154 in Vietnam, is a COVID-19 vaccine candidate developed by Arcturus Therapeutics. For its development, Arcturus collaborated with Vinbiocare, a Vietnamese company, for support with clinical trials and manufacturing. The vaccine was authorised in Japan in November 2023.

COVID-19 vaccine clinical research uses clinical research to establish the characteristics of COVID-19 vaccines. These characteristics include efficacy, effectiveness, and safety. As of November 2022, 40 vaccines are authorized by at least one national regulatory authority for public use:

Noora is a COVID-19 vaccine candidate developed by Baqiyatallah University of Medical Sciences in collaboration with Plasma Darman Sarv Sepid Co. in Iran. Introduced in June 2021, it was announced as having "successfully passed the first phase of its clinical trial" two months later.

Soberana Plus, technical name FINLAY-FR-1A, is a COVID-19 candidate vaccine produced by the Finlay Institute, a Cuban epidemiological research institute.

S-268019-b is a protein subunit COVID-19 vaccine candidate developed by Shionogi.

LYB001 is a COVID-19 vaccine candidate developed by Yantai Patronus Biotech Co., Ltd.







