Related Research Articles

Sinovac Biotech Ltd. is a Chinese biopharmaceutical company based in Haidian District, Beijing that focuses on the research, development, manufacture, and commercialization of vaccines that protect against human infectious diseases. The company was listed on the Nasdaq but the exchange halted Sinovac's trading in February 2019 due to a proxy fight. The company has faced bribery probes in China. Its COVID-19 vaccine was also the target of a covert disinformation campaign by the US government.

A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID‑19).
The COVID-19 pandemic in Guatemala is a part of the worldwide pandemic of coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus 2. The virus was confirmed to have reached Guatemala in March 2020.
The COVID-19 pandemic in Haiti is part of the worldwide pandemic of coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus 2. The virus was confirmed to have reached Haiti in March 2020. The index case was in Port-au-Prince. As of 14 September 2021, there are 21,178 total confirmed cases, 1,184 active cases, about 32,000 suspected cases, with 591 deaths and 8,657 recoveries. Haiti has administered 50,624 doses of the COVID-19 vaccination.

The Moderna COVID‑19 vaccine, sold under the brand name Spikevax, is a COVID-19 vaccine developed by the American company Moderna, the United States National Institute of Allergy and Infectious Diseases (NIAID), and the Biomedical Advanced Research and Development Authority (BARDA). Depending on the jurisdiction, it is authorized for use in humans aged six months, twelve years, or eighteen years and older. It provides protection against COVID-19, which is caused by infection by the SARS-CoV-2 virus.
The first four cases of the COVID-19 pandemic in Jabalpur, Madhya Pradesh were confirmed on March 20, 2020. As of August 14, 2021, Madhya Pradesh has confirmed a total of 791,998 cases, and has recorded 10,514 deaths.

The Jenner Institute is a research institute on the Old Road Campus in Headington, east Oxford, England. It was formed in November 2005 through a partnership between the University of Oxford and the UK Institute for Animal Health. It is associated with the Nuffield Department of Medicine, in the Medical Sciences Division of Oxford University. The institute receives charitable support from the Jenner Vaccine Foundation.

Operation Warp Speed (OWS) was a public–private partnership initiated by the United States government to facilitate and accelerate the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics. The first news report of Operation Warp Speed was on April 29, 2020, and the program was officially announced on May 15, 2020. It was headed by Moncef Slaoui from May 2020 to January 2021 and by David A. Kessler from January to February 2021. At the end of February 2021, Operation Warp Speed was transferred into the responsibilities of the White House COVID-19 Response Team.

The Oxford–AstraZeneca COVID‑19 vaccine, sold under the brand names Covishield and Vaxzevria among others, is a viral vector vaccine for the prevention of COVID-19. It was developed in the United Kingdom by Oxford University and British-Swedish company AstraZeneca, using as a vector the modified chimpanzee adenovirus ChAdOx1. The vaccine is given by intramuscular injection. Studies carried out in 2020 showed that the efficacy of the vaccine is 76.0% at preventing symptomatic COVID-19 beginning at 22 days following the first dose and 81.3% after the second dose. A study in Scotland found that, for symptomatic COVID-19 infection after the second dose, the vaccine is 81% effective against the Alpha variant and 61% against the Delta variant.

Israel's COVID-19 vaccination programme, officially named "Give a Shoulder", began on 19 December 2020, and has been praised for its speed, having given twenty percent of the Israeli population the first dose of the vaccines' two dose regimen in the span of three weeks.

The Janssen COVID‑19 vaccine, (Ad26.COV2.S) sold under the brand name Jcovden, is a COVID‑19 vaccine that was developed by Janssen Vaccines in Leiden, Netherlands, and its Belgian parent company Janssen Pharmaceuticals, a subsidiary of American company Johnson & Johnson.

India began administration of COVID-19 vaccines on 16 January 2021. As of 4 March 2023, India has administered over 2.2 billion doses overall, including first, second and precautionary (booster) doses of the currently approved vaccines. In India, 95% of the eligible population (12+) has received at least one shot, and 88% of the eligible population (12+) is fully vaccinated.

As of 12 August 2024, 13.53 billion COVID-19 vaccine doses have been administered worldwide, with 70.6 percent of the global population having received at least one dose. While 4.19 million vaccines were then being administered daily, only 22.3 percent of people in low-income countries had received at least a first vaccine by September 2022, according to official reports from national health agencies, which are collated by Our World in Data.

SARS-CoV-2, the virus that causes COVID-19, was isolated in late 2019. Its genetic sequence was published on 11 January 2020, triggering an urgent international response to prepare for an outbreak and hasten the development of a preventive COVID-19 vaccine. Since 2020, vaccine development has been expedited via unprecedented collaboration in the multinational pharmaceutical industry and between governments. By June 2020, tens of billions of dollars were invested by corporations, governments, international health organizations, and university research groups to develop dozens of vaccine candidates and prepare for global vaccination programs to immunize against COVID‑19 infection. According to the Coalition for Epidemic Preparedness Innovations (CEPI), the geographic distribution of COVID‑19 vaccine development shows North American entities to have about 40% of the activity, compared to 30% in Asia and Australia, 26% in Europe, and a few projects in South America and Africa.

Post-vaccination embolic and thrombotic events, termed vaccine-induced immune thrombotic thrombocytopenia (VITT), vaccine-induced prothrombotic immune thrombocytopenia (VIPIT), thrombosis with thrombocytopenia syndrome (TTS), vaccine-induced immune thrombocytopenia and thrombosis (VITT), or vaccine-associated thrombotic thrombocytopenia (VATT), are rare types of blood clotting syndromes that were initially observed in a number of people who had previously received the Oxford–AstraZeneca COVID‑19 vaccine (AZD1222) during the COVID‑19 pandemic. It was subsequently also described in the Janssen COVID‑19 vaccine, leading to the suspension of its use until its safety had been reassessed. On 5 May 2022 the FDA posted a bulletin limiting the use of the Janssen Vaccine to very specific cases due to further reassessment of the risks of TTS, although the FDA also stated in the same bulletin that the benefits of the vaccine outweigh the risks.
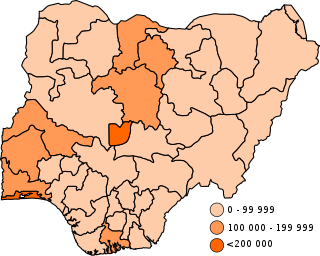
COVID-19 vaccination in Nigeria is an ongoing immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country. Vaccination began on 5 March 2021. As of 28 February 2022, 17,914,944 people have received their first dose a COVID-19 vaccine, and 8,197,832 have received their second dose.

COVID-19 vaccination in Kazakhstan is an ongoing immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.

A COVID-19 vaccine card is a record often given to those who have received a COVID-19 vaccine showing information such as the date(s) one has received the shot(s) and the brand of vaccine one has received, sometimes including the lot number. The card also contains information identifying the recipient and the location where the shot was given. Depending on the country, it could serve as an official document verifying one has received vaccination, which could be required by some institutions, such as a school or workplace, when boarding a cruise ship, or when crossing an international border, as proof that one has been vaccinated.

A vaccine passport or proof of vaccination is an immunity passport employed as a credential in countries and jurisdictions as part of efforts to control the COVID-19 pandemic via vaccination. A vaccine passport is typically issued by a government or health authority, and usually consists of a digital or printed record. Some credentials may include a scannable QR code, which can also be provisioned via mobile app. It may or may not use a COVID-19 vaccine card as a basis of authentication.
References
- ↑ "Haiti fights large COVID-19 spike as it awaits vaccines". AP NEWS. 2021-06-09. Archived from the original on 2021-06-19. Retrieved 20 June 2021.
- ↑ "U.S. to send vaccines to Latin America, Caribbean as COVID cases and deaths are surging". Archived from the original on 21 June 2021. Retrieved 20 June 2021.