
Adenoviruses are medium-sized, nonenveloped viruses with an icosahedral nucleocapsid containing a double-stranded DNA genome. Their name derives from their initial isolation from human adenoids in 1953.
The Lazzaro Spallanzani National Institute for Infectious Diseases is an infectious disease hospital in the Italian city of Rome. The institute is named for the eighteenth-century Italian biologist Lazzaro Spallanzani.

A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID‑19).
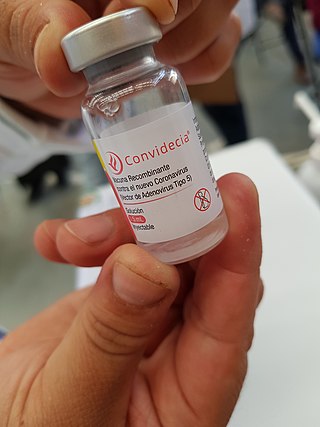
AD5-nCOV, trade-named Convidecia, is a single-dose viral vector vaccine for COVID-19 that is also used as an inhaled booster. It was developed by CanSino Biologics, with Phase III trials conducted in Argentina, Chile, Mexico, Pakistan, Russia, and Saudi Arabia with 40,000 participants.

The Oxford–AstraZeneca COVID‑19 vaccine, sold under the brand names Covishield and Vaxzevria among others, is a viral vector vaccine for prevention of COVID-19. Developed in the United Kingdom by Oxford University and British-Swedish company AstraZeneca, using as a vector the modified chimpanzee adenovirus ChAdOx1. The vaccine is given by intramuscular injection. Studies carried out in 2020 showed that the efficacy of the vaccine is 76.0% at preventing symptomatic COVID-19 beginning at 22 days following the first dose, and 81.3% after the second dose. A study in Scotland found that, for symptomatic COVID-19 infection after the second dose, the vaccine is 81% effective against the Alpha variant, and 61% against the Delta variant.

Valneva COVID-19 vaccine is a COVID-19 vaccine developed by French biotechnology company Valneva SE in collaboration with the American biopharmaceutical company Dynavax Technologies.

The Janssen COVID‑19 vaccine, sold under the brand name Jcovden, is a COVID‑19 vaccine that was developed by Janssen Vaccines in Leiden, Netherlands, and its Belgian parent company Janssen Pharmaceuticals, a subsidiary of American company Johnson & Johnson.

SARS-CoV-2, the virus that causes COVID-19, was isolated in late 2019. Its genetic sequence was published on 11 January 2020, triggering the urgent international response to prepare for an outbreak and hasten development of a preventive COVID-19 vaccine. Since 2020, vaccine development has been expedited via unprecedented collaboration in the multinational pharmaceutical industry and between governments. By June 2020, tens of billions of dollars were invested by corporations, governments, international health organizations, and university research groups to develop dozens of vaccine candidates and prepare for global vaccination programs to immunize against COVID‑19 infection. According to the Coalition for Epidemic Preparedness Innovations (CEPI), the geographic distribution of COVID‑19 vaccine development shows North American entities to have about 40% of the activity, compared to 30% in Asia and Australia, 26% in Europe, and a few projects in South America and Africa.

ZyCoV-D is a DNA plasmid-based COVID-19 vaccine developed by Indian pharmaceutical company Cadila Healthcare, with support from the Biotechnology Industry Research Assistance Council. It is approved for emergency use in India.

ARCT-021, also known as LUNAR-COV19, is a COVID-19 vaccine candidate developed by Arcturus Therapeutics.

iNCOVACC is an intranasal COVID-19 vaccine candidate developed by Bharat Biotech, American company Precision Virologics and the Washington University School of Medicine in St Louis, Missouri, United States.

The MVC COVID-19 vaccine, designated MVC-COV1901 and also known as the Medigen COVID-19 vaccine, is a protein subunit COVID-19 vaccine developed by Medigen Vaccine Biologics Corporation in Taiwan, American company Dynavax Technologies, and the U.S. National Institutes of Health.

A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material (DNA) that can be transcribed by the recipient's host cells as mRNA coding for a desired protein, or antigen, to elicit an immune response. As of April 2021, six viral vector vaccines, four COVID-19 vaccines and two Ebola vaccines, have been authorized for use in humans.
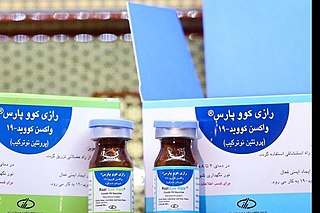
Razi Cov Pars is a COVID-19 vaccine developed by the Iranian Razi Vaccine and Serum Research Institute.

COVAX-19 is the result of a collaboration between Vaxine and CinnaGen, a private company with operations in the Middle East. COVAX-19 is a recombinant protein-based COVID-19 vaccine developed by South Australian-based biotech company Vaxine. It is under clinical trial in collaboration with the Iranian company CinnaGen.

AdCLD-CoV19 is a COVID-19 vaccine candidate developed by Cellid Co, a company from South Korea.

COVID-19 vaccine clinical research uses clinical research to establish the characteristics of COVID-19 vaccines. These characteristics include efficacy, effectiveness and safety. As of November 2022, 40 vaccines are authorized by at least one national regulatory authority for public use:

ReCOV is a COVID-19 vaccine candidate developed by Jiangsu Rec-Biotechnology Co Ltd.

AKS-452 is a COVID-19 vaccine candidate developed by Akston Biosciences.

SCTV01C is a COVID-19 vaccine candidate developed by Sinocelltech.









