
The Mantoux test or Mendel–Mantoux test is a tool for screening for tuberculosis (TB) and for tuberculosis diagnosis. It is one of the major tuberculin skin tests used around the world, largely replacing multiple-puncture tests such as the tine test. The Heaf test, a form of tine test, was used until 2005 in the UK, when it was replaced by the Mantoux test. The Mantoux test is endorsed by the American Thoracic Society and Centers for Disease Control and Prevention. It was also used in the USSR and is now prevalent in most of the post-Soviet states, although Soviet mantoux produced many false positives due to children's allergic reaction.

A syringe is a simple reciprocating pump consisting of a plunger that fits tightly within a cylindrical tube called a barrel. The plunger can be linearly pulled and pushed along the inside of the tube, allowing the syringe to take in and expel liquid or gas through a discharge orifice at the front (open) end of the tube. The open end of the syringe may be fitted with a hypodermic needle, a nozzle or tubing to direct the flow into and out of the barrel. Syringes are frequently used in clinical medicine to administer injections, infuse intravenous therapy into the bloodstream, apply compounds such as glue or lubricant, and draw/measure liquids. There are also prefilled syringes.

An autoinjector is a medical device designed to deliver a dose of a particular drug. The injectors were initially designed to overcome the hesitation associated with self-administration of the needle-based drug delivery device.

A hypodermic needle, one of a category of medical tools which enter the skin, called sharps, is a very thin, hollow tube with one sharp tip. It is commonly used with a syringe, a hand-operated device with a plunger, to inject substances into the body or extract fluids from the body. Large-bore hypodermic intervention is especially useful in catastrophic blood loss or treating shock.

In pharmacology and toxicology, a route of administration is the way by which a drug, fluid, poison, or other substance is taken into the body.

Subcutaneous administration is the insertion of medications beneath the skin either by injection or infusion.

Intramuscular injection, often abbreviated IM, is the injection of a substance into a muscle. In medicine, it is one of several methods for parenteral administration of medications. Intramuscular injection may be preferred because muscles have larger and more numerous blood vessels than subcutaneous tissue, leading to faster absorption than subcutaneous or intradermal injections. Medication administered via intramuscular injection is not subject to the first-pass metabolism effect which affects oral medications.
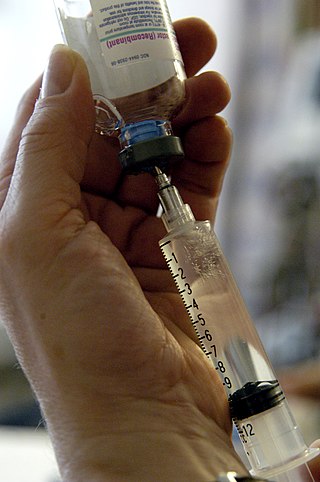
An injection is the act of administering a liquid, especially a drug, into a person's body using a needle and a syringe. An injection is considered a form of parenteral drug administration; it does not involve absorption in the digestive tract. This allows the medication to be absorbed more rapidly and avoid the first pass effect. There are many types of injection, which are generally named after the body tissue the injection is administered into. This includes common injections such as subcutaneous, intramuscular, and intravenous injections, as well as less common injections such as intraperitoneal, intraosseous, intracardiac, intraarticular, and intracavernous injections.

A transdermal patch is a medicated adhesive patch that is placed on the skin to deliver a specific dose of medication through the skin and into the bloodstream. An advantage of a transdermal drug delivery route over other types of medication delivery is that the patch provides a controlled release of the medication into the patient, usually through either a porous membrane covering a reservoir of medication or through body heat melting thin layers of medication embedded in the adhesive. The main disadvantage to transdermal delivery systems stems from the fact that the skin is a very effective barrier; as a result, only medications whose molecules are small enough to penetrate the skin can be delivered by this method. The first commercially available prescription patch was approved by the U.S. Food and Drug Administration in December 1979. These patches administered scopolamine for motion sickness.

A jet injector is a type of medical injecting syringe device used for a method of drug delivery known as jet injection. A narrow, high-pressure stream of liquid is made to penetrate the outermost layer of the skin to deliver medication to targeted underlying tissues of the epidermis or dermis, fat, or muscle.

Drug injection is a method of introducing a drug into the bloodstream via a hollow hypodermic needle, which is pierced through the skin into the body. Intravenous therapy, a form of drug injection, is universally practiced in modernized medical care. As of 2004, there were 13.2 million people worldwide who self-administered injection drugs outside of medical supervision, of which 22% are from developed countries.
Dosage forms are pharmaceutical drug products in the form in which they are marketed for use, with a specific mixture of active ingredients and inactive components (excipients), in a particular configuration, and apportioned into a particular dose. For example, two products may both be amoxicillin, but one is in 500 mg capsules and another is in 250 mg chewable tablets. The term unit dose can also sometimes encompass non-reusable packaging as well, although the FDA distinguishes that by unit-dose "packaging" or "dispensing". Depending on the context, multi(ple) unit dose can refer to distinct drug products packaged together, or to a single drug product containing multiple drugs and/or doses. The term dosage form can also sometimes refer only to the pharmaceutical formulation of a drug product's constituent drug substance(s) and any blends involved, without considering matters beyond that. Because of the somewhat vague boundaries and unclear overlap of these terms and certain variants and qualifiers within the pharmaceutical industry, caution is often advisable when conversing with someone who may be unfamiliar with another person's use of the term.
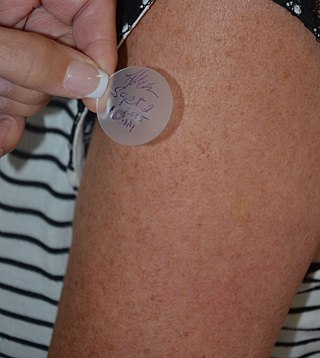
Transdermal is a route of administration wherein active ingredients are delivered across the skin for systemic distribution. Examples include transdermal patches used for medicine delivery. The drug is administered in the form of a patch or ointment that delivers the drug into the circulation for systemic effect.
In medicine, a bolus is the administration of a discrete amount of medication, drug, or other compound within a specific time, generally 1–30 minutes, to raise its concentration in blood to an effective level. The administration can be given by injection: intravenously, intramuscularly, intrathecally, subcutaneously, or by inhalation. The article on routes of administration provides more information, as the preceding list of ROAs is not exhaustive.
Skin popping is a route of administration of street drugs where they are injected or deposited under the skin. It is usually a depot injection, either subcutaneous or intradermal, and not an intramuscular injection. After deposition, the drug then diffuses slowly from the depot into the capillary networks, where it enters circulation. Skin popping is distinct from intravenous injection in that the latter deposits the drug directly into the bloodstream via a vein. Nonetheless, it is included with IV injection in the category of injection drug use because both involve injection, both are often done with the same drugs, and both carry many of the same risks. Higher-potency prescription opioids, such as morphine, fentanyl, or meperidine can be injected subcutaneously, as can cocaine. Skin popping increases the duration of the high one gets from drugs such as cocaine. The sites where skin popping with cocaine has been performed have an area of central pallor surrounded by bruising (ecchymosis). This pattern is due to the vasoconstrictive properties of cocaine acting locally at the injection site with hemorrhage occurring in the surrounding tissue. Skin popping puts one at risk for developing secondary amyloid associated (AA) amyloidosis. Tetanus has also been associated with skin-popping as has botulism.

An injection port is a medical device used for the administration of insulin or other physician-approved medicine into the subcutaneous tissue. The device is similar to infusion sets used by insulin pumps, except it is configured to receive a syringe instead of a tubing system. An injection port is usually a disposable device applied by the patient and worn for period of 3–5 days. When giving shots via an injection port, the needle stays above the surface of the skin. Medication is delivered via a short soft cannula. An injection port can be used in conjunction with multiple daily injections of insulin by people with diabetes. It can also be used for the subcutaneous administration of any other physician prescribed medication.
Injection site reactions (ISRs) are reactions that occur at the site of injection of a drug. They may be mild or severe and may or may not require medical intervention. Some reactions may appear immediately after injection, and some may be delayed. Such reactions can occur with subcutaneous, intramuscular, or intravenous administration.

Microneedles or Microneedle patches or Microarray patches are micron-scaled medical devices used to administer vaccines, drugs, and other therapeutic agents. While microneedles were initially explored for transdermal drug delivery applications, their use has been extended for the intraocular, vaginal, transungual, cardiac, vascular, gastrointestinal, and intracochlear delivery of drugs. Microneedles are constructed through various methods, usually involving photolithographic processes or micromolding. These methods involve etching microscopic structure into resin or silicon in order to cast microneedles. Microneedles are made from a variety of material ranging from silicon, titanium, stainless steel, and polymers. Some microneedles are made of a drug to be delivered to the body but are shaped into a needle so they will penetrate the skin. The microneedles range in size, shape, and function but are all used as an alternative to other delivery methods like the conventional hypodermic needle or other injection apparatus.

An injector pen is a device used for injecting medication under the skin. First introduced in the 1980s, injector pens are designed to make injectable medication easier and more convenient to use, thus increasing patient adherence. The primary difference between injector pens and traditional vial and syringe administration is the easier use of an injector pen by people with low dexterity, poor vision, or who need portability to administer medicine on time. Injector pens also decrease the fear or adversity towards self-injection of medications, which increases the likelihood that a person takes the medication.
Microneedles (MNs) are medical tools used for microneedling, primarily in drug delivery, disease diagnosis, and collagen induction therapy. Known for their minimally invasive and precise nature, MNs consist of arrays of micro-sized needles ranging from 25μm to 2000μm. Although the concept of microneedling was first introduced in the 1970s, its popularity has surged due to its effectiveness in drug delivery and its cosmetic benefits.























