A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.

In polymer chemistry, polymerization, or polymerisation, is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them.
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar. Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups.
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer. The reactive center can be radical, anionic or cationic.
In polymer chemistry, emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomers, and surfactants. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer are emulsified in a continuous phase of water. Water-soluble polymers, such as certain polyvinyl alcohols or hydroxyethyl celluloses, can also be used to act as emulsifiers/stabilizers. The name "emulsion polymerization" is a misnomer that arises from a historical misconception. Rather than occurring in emulsion droplets, polymerization takes place in the latex/colloid particles that form spontaneously in the first few minutes of the process. These latex particles are typically 100 nm in size, and are made of many individual polymer chains. The particles are prevented from coagulating with each other because each particle is surrounded by the surfactant ('soap'); the charge on the surfactant repels other particles electrostatically. When water-soluble polymers are used as stabilizers instead of soap, the repulsion between particles arises because these water-soluble polymers form a 'hairy layer' around a particle that repels other particles, because pushing particles together would involve compressing these chains.
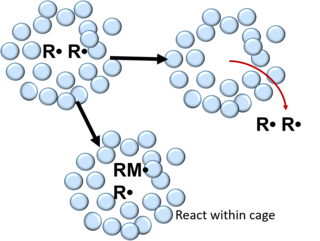
In chemistry, the cage effect (also known as geminate recombination) describes how the properties of a molecule are affected by its surroundings. First introduced by James Franck and Eugene Rabinowitch in 1934, the cage effect suggests that instead of acting as an individual particle, molecules in solvent are more accurately described as an encapsulated particle. The encapsulated molecules or radicals are called cage pairs or geminate pairs. In order to interact with other molecules, the caged particle must diffuse from its solvent cage. The typical lifetime of a solvent cage is 10-11 seconds. Many manifestations of the cage effect exist.

Chain-growth polymerization (AE) or chain-growth polymerisation (BE) is a polymerization technique where monomer molecules add onto the active site on a growing polymer chain one at a time. There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics.

In polymer chemistry, step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally-occurring and some synthetic polymers are produced by step-growth polymerization, e.g. polyesters, polyamides, polyurethanes, etc. Due to the nature of the polymerization mechanism, a high extent of reaction is required to achieve high molecular weight. The easiest way to visualize the mechanism of a step-growth polymerization is a group of people reaching out to hold their hands to form a human chain—each person has two hands. There also is the possibility to have more than two reactive sites on a monomer: In this case branched polymers production take place.

In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks. Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain.
In polymer chemistry, anionic addition polymerization is a form of chain-growth polymerization or addition polymerization that involves the polymerization of monomers initiated with anions. The type of reaction has many manifestations, but traditionally vinyl monomers are used. Often anionic polymerization involves living polymerizations, which allows control of structure and composition.

Atom transfer radical polymerization (ATRP) is an example of a reversible-deactivation radical polymerization. Like its counterpart, ATRA, or atom transfer radical addition, ATRP is a means of forming a carbon-carbon bond with a transition metal catalyst. Polymerization from this method is called atom transfer radical addition polymerization (ATRAP). As the name implies, the atom transfer step is crucial in the reaction responsible for uniform polymer chain growth. ATRP was independently discovered by Mitsuo Sawamoto and by Krzysztof Matyjaszewski and Jin-Shan Wang in 1995.

Reversible addition−fragmentation chain-transfer or RAFT polymerization is one of several kinds of reversible-deactivation radical polymerization. It makes use of a chain-transfer agent (CTA) in the form of a thiocarbonylthio compound to afford control over the generated molecular weight and polydispersity during a free-radical polymerization. Discovered at the Commonwealth Scientific and Industrial Research Organisation (CSIRO) of Australia in 1998, RAFT polymerization is one of several living or controlled radical polymerization techniques, others being atom transfer radical polymerization (ATRP) and nitroxide-mediated polymerization (NMP), etc. RAFT polymerization uses thiocarbonylthio compounds, such as dithioesters, thiocarbamates, and xanthates, to mediate the polymerization via a reversible chain-transfer process. As with other controlled radical polymerization techniques, RAFT polymerizations can be performed under conditions that favor low dispersity and a pre-chosen molecular weight. RAFT polymerization can be used to design polymers of complex architectures, such as linear block copolymers, comb-like, star, brush polymers, dendrimers and cross-linked networks.
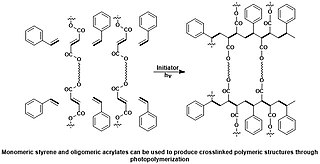
A photopolymer or light-activated resin is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing.
In polymer chemistry, chain transfer is a polymerization reaction by which the activity of a growing polymer chain is transferred to another molecule:

In polymer chemistry, autoacceleration is a dangerous reaction behavior that can occur in free-radical polymerization systems. It is due to the localized increases in viscosity of the polymerizing system that slow termination reactions. The removal of reaction obstacles therefore causes a rapid increase in the overall rate of reaction, leading to possible reaction runaway and altering the characteristics of the polymers produced. It is also known as the Trommsdorff–Norrish effect after German chemist Johann Trommsdorff and British chemist Ronald G.W. Norrish.
In polymer chemistry, the kinetic chain length of a polymer is the average number of units called monomers added to a growing chain during chain-growth polymerization. During this process, a polymer chain is formed when monomers are bonded together to form long chains known as polymers. Kinetic chain length is defined as the average number of monomers that react with an active center such as a radical from initiation to termination.
Living cationic polymerization is a living polymerization technique involving cationic propagating species. It enables the synthesis of very well defined polymers and of polymers with unusual architecture such as star polymers and block copolymers and living cationic polymerization is therefore as such of commercial and academic interest.

In polymer chemistry, reversible-deactivation radical polymerizations (RDRPs) are members of the class of reversible-deactivation polymerizations which exhibit much of the character of living polymerizations, but cannot be categorized as such as they are not without chain transfer or chain termination reactions. Several different names have been used in literature, which are:
In polymer chemistry, ionic polymerization is a chain-growth polymerization in which active centers are ions or ion pairs. It can be considered as an alternative to radical polymerization, and may refer to anionic polymerization or cationic polymerization.
Polyoxetane (POX), or poly(oxetane), is synthetic organic heteroatomic thermoplastic polymer with molecular formula (–OCH2CH2CH2–)n. It is polymerized from oxetane monomer, which is a four-membered cyclic ether.



































