
Syngas, or synthesis gas, is a fuel gas mixture consisting primarily of hydrogen, carbon monoxide, and very often some carbon dioxide. The name comes from its use as intermediates in creating synthetic natural gas (SNG) and for producing ammonia or methanol. Syngas is combustible and can be used as a fuel of internal combustion engines. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII. However, it has less than half the energy density of natural gas.

The German Aerospace Center, abbreviated DLR, is the national center for aerospace, energy and transportation research of Germany. Its headquarters are located in Cologne and it has multiple other locations throughout Germany. The DLR is engaged in a wide range of research and development projects in national and international partnerships. In addition to conducting its own research projects, DLR also acts as the German space agency. As such, it is responsible for planning and implementing the German space programme on behalf of the German federal government. As a project management agency, DLR also coordinates and answers the technical and organisational implementation of projects funded by a number of German federal ministries.
The hydrogen economy is using hydrogen to decarbonize economic sectors which are hard to electrify, essentially, the "hard-to-abate" sectors such as cement, steel, long-haul transport etc. In order to phase out fossil fuels and limit climate change, hydrogen can be created from water using renewable sources such as wind and solar, and its combustion only releases water vapor to the atmosphere.

High-temperature electrolysis is a technology for producing hydrogen from water at high temperatures.
Artificial photosynthesis is a chemical process that biomimics the natural process of photosynthesis to convert sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term artificial photosynthesis is commonly used to refer to any scheme for capturing and storing the energy from sunlight in the chemical bonds of a fuel. Photocatalytic water splitting converts water into hydrogen and oxygen and is a major research topic of artificial photosynthesis. Light-driven carbon dioxide reduction is another process studied that replicates natural carbon fixation.

Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:

Cerium(IV) oxide, also known as ceric oxide, ceric dioxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare-earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2. It is an important commercial product and an intermediate in the purification of the element from the ores. The distinctive property of this material is its reversible conversion to a non-stoichiometric oxide.

The sulfur–iodine cycle is a three-step thermochemical cycle used to produce hydrogen.

In space exploration, in situ resource utilization (ISRU) is the practice of collection, processing, storing and use of materials found or manufactured on other astronomical objects that replace materials that would otherwise be brought from Earth.
Hydrogen production is the family of industrial methods for generating hydrogen gas. As of 2020, the majority of hydrogen (∼95%) is produced from fossil fuels by steam reforming of natural gas and other light hydrocarbons, partial oxidation of heavier hydrocarbons, and coal gasification. Other methods of hydrogen production include biomass gasification, zero-CO2-emission methane pyrolysis, and electrolysis of water. The latter processes, methane pyrolysis as well as water electrolysis can be done directly with any source of electricity, such as solar power.

The copper–chlorine cycle is a four-step thermochemical cycle for the production of hydrogen. The Cu–Cl cycle is a hybrid process that employs both thermochemical and electrolysis steps. It has a maximum temperature requirement of about 530 degrees Celsius.

The hybrid sulfur cycle (HyS) is a two-step water-splitting process intended to be used for hydrogen production. Based on sulfur oxidation and reduction, it is classified as a hybrid thermochemical cycle because it uses an electrochemical reaction for one of the two steps. The remaining thermochemical step is shared with the sulfur-iodine cycle.

For chemical reactions, the iron oxide cycle (Fe3O4/FeO) is the original two-step thermochemical cycle proposed for use for hydrogen production. It is based on the reduction and subsequent oxidation of iron ions, particularly the reduction and oxidation between Fe3+ and Fe2+. The ferrites, or iron oxide, begins in the form of a spinel and depending on the reaction conditions, dopant metals and support material forms either Wüstites or different spinels.
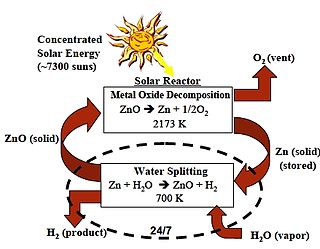
For chemical reactions, the zinc–zinc oxide cycle or Zn–ZnO cycle is a two step thermochemical cycle based on zinc and zinc oxide for hydrogen production with a typical efficiency around 40%.

The cerium(IV) oxide–cerium(III) oxide cycle or CeO2/Ce2O3 cycle is a two-step thermochemical process that employs cerium(IV) oxide and cerium(III) oxide for hydrogen production. The cerium-based cycle allows the separation of H2 and O2 in two steps, making high-temperature gas separation redundant.

Cerium(III) oxide, also known as cerium oxide, cerium trioxide, cerium sesquioxide, cerous oxide or dicerium trioxide, is an oxide of the rare-earth metal cerium. It has chemical formula Ce2O3 and is gold-yellow in color.
Thermochemical cycles combine solely heat sources (thermo) with chemical reactions to split water into its hydrogen and oxygen components. The term cycle is used because aside of water, hydrogen and oxygen, the chemical compounds used in these processes are continuously recycled.
A solar fuel is a synthetic chemical fuel produced from solar energy. Solar fuels can be produced through photochemical, photobiological, thermochemical, and electrochemical reactions. Light is used as an energy source, with solar energy being transduced to chemical energy, typically by reducing protons to hydrogen, or carbon dioxide to organic compounds.
Power-to-gas is a technology that uses electric power to produce a gaseous fuel. When using surplus power from wind generation, the concept is sometimes called windgas.
E-diesel is a synthetic diesel fuel created by Audi for use in automobiles. Currently, e-diesel is created by an Audi research facility in partnership with a company named Sunfire. The fuel is created from carbon dioxide, water, and electricity with a process powered by renewable energy sources to create a liquid energy carrier called blue crude which is then refined to generate e-diesel. E-diesel is considered to be a carbon-neutral fuel as it does not extract new carbon and the energy sources to drive the process are from carbon-neutral sources. As of April 2015, an Audi A8 driven by Federal Minister of Education and Research in Germany is using the e-diesel fuel.












