Related Research Articles

Sildenafil, sold under the brand name Viagra, among others, is a medication used to treat erectile dysfunction and pulmonary arterial hypertension. It is also sometimes used off-label for the treatment of certain symptoms in secondary Raynaud's phenomenon. It is unclear if it is effective for treating sexual dysfunction in females. It can be taken orally, intravenously, or through the sublingual route. Onset when taken orally is typically within twenty minutes and lasts for about two hours.

Medical cannabis, medicinal cannabis or medical marijuana (MMJ), refers to cannabis products and cannabinoid molecules that are prescribed by physicians for their patients. The use of cannabis as medicine has a long history, but has not been as rigorously tested as other medicinal plants due to legal and governmental restrictions, resulting in limited clinical research to define the safety and efficacy of using cannabis to treat diseases.

Tadalafil, sold under the brand name Cialis among others, is a medication used to treat erectile dysfunction, benign prostatic hyperplasia, and pulmonary arterial hypertension. It is taken by mouth. Onset is typically within half an hour and the duration is up to 36 hours.

Yohimbine, also known as quebrachine, is an indole alkaloid derived from the bark of the African tree Pausinystalia johimbe; also from the bark of the unrelated South American tree Aspidosperma quebracho-blanco. Yohimbine is an α2-adrenergic receptor antagonist, and has been used in a variety of research projects. It is a veterinary drug used to reverse sedation in dogs and deer.

Mitragyna speciosa is a tropical evergreen tree of the Rubiaceae family native to Southeast Asia. It is indigenous to Cambodia, Thailand, Indonesia, Malaysia, Myanmar, and Papua New Guinea, where its leaves, known as "kratom" have been used in herbal medicine since at least the 19th century. They have also historically been consumed via chewing, smoking, and as a tea. Kratom has opioid-like properties and some stimulant-like effects. As of 2018, the efficacy and safety of kratom are unclear. In 2019, the United States Food and Drug Administration (FDA) stated that there is no evidence that kratom is safe or effective for treating any condition. Some people take it for managing chronic pain, for treating opioid withdrawal symptoms, or for recreational purposes. The onset of effects typically begins within five to ten minutes and lasts for two to five hours.

The regulation of therapeutic goods, defined as drugs and therapeutic devices, varies by jurisdiction. In some countries, such as the United States, they are regulated at the national level by a single agency. In other jurisdictions they are regulated at the state level, or at both state and national levels by various bodies, as in Australia.

Cannabidiol (CBD) is a phytocannabinoid, one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. Medically, it is an anticonvulsant used to treat multiple forms of epilepsy. It was discovered in 1940 and, as of 2022, clinical research on CBD included studies related to the treatment of anxiety, addiction, psychosis, movement disorders, and pain, but there is insufficient high-quality evidence that CBD is effective for these conditions. CBD is sold as an herbal dietary supplement and promoted with yet unproven claims of particular therapeutic effects.

Ranitidine, previously sold under the brand name Zantac among others, is a medication used to decrease stomach acid production. It was commonly used in treatment of peptic ulcer disease, gastroesophageal reflux disease, and Zollinger–Ellison syndrome. It can be given by mouth, injection into a muscle, or injection into a vein.

Budesonide/formoterol, sold under the brand name Symbicort among others, is a fixed-dose combination medication used in the management of asthma or chronic obstructive pulmonary disease (COPD). It contains budesonide, a steroid; and formoterol, a long-acting β2-agonist (LABA). The product monograph does not support its use for sudden worsening or treatment of active bronchospasm. However, a 2020 review of the literature does support such use. It is used by breathing in the medication.

The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).
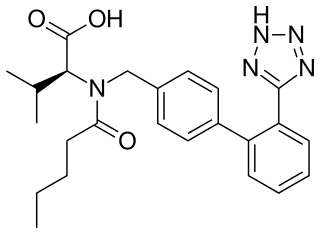
Valsartan, sold under the brand name Diovan among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It belongs to a class of medications referred to as angiotensin II receptor blockers (ARBs). It is a reasonable initial treatment for high blood pressure. It is taken by mouth.

The Standard for the Uniform Scheduling of Medicines and Poisons (SUSMP), also known as the Poisons Standard for short, is an Australian legislative instrument produced by the Therapeutic Goods Administration (TGA). Before 2010, it was known as the Standard for the Uniform Scheduling of Drugs and Poisons (SUSDP). The SUSMP classifies drugs and poisons into different Schedules signifying the degree of control recommended to be exercised over their availability to the public. As of 2024, the most recent version is the Therapeutic Goods Instrument 2024.

N-Nitrosodimethylamine (NDMA), also known as dimethylnitrosamine (DMN), is an organic compound with the formula (CH3)2NNO. It is one of the simplest members of a large class of nitrosamines. It is a volatile yellow oil. NDMA has attracted wide attention as being highly hepatotoxic and a known carcinogen in laboratory animals.
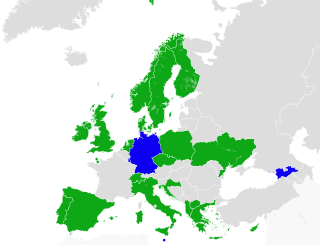
Cannabis in the United Kingdom is illegal for recreational use and is classified as a Class B drug. In 2004, the United Kingdom made cannabis a Class C drug with less severe penalties, but it was moved back to Class B in 2009. Medical use of cannabis, when prescribed by a registered specialist doctor, was legalised in November 2018.
A counterfeit medication or a counterfeit drug is a medication or pharmaceutical item which is produced and sold with the intent to deceptively represent its origin, authenticity, or effectiveness. A counterfeit drug may contain inappropriate quantities of active ingredients, or none, may be improperly processed within the body, may contain ingredients that are not on the label, or may be supplied with inaccurate or fake packaging and labeling.

Valsartan/hydrochlorothiazide, sold under the brand name Diovan HCT among others, is a medication used to treat high blood pressure when valsartan is not sufficient. It is a combination of valsartan, an angiotensin receptor blocker with hydrochlorothiazide, a diuretic. It is taken by mouth.

Aurobindo Pharma Limited is an Indian multinational pharmaceutical manufacturing company headquartered in HITEC City, Hyderabad. The company manufactures generic pharmaceuticals and active pharmaceutical ingredients. The company's area of activity includes six major therapeutic and product areas: antibiotics, anti-retrovirals, cardiovascular products, central nervous system products, gastroenterologicals, and anti-allergics. The company markets these products in over 125 countries. Its marketing partners include AstraZeneca and Pfizer.

Cannabis in Cyprus is illegal for recreational use but legal for medical use.
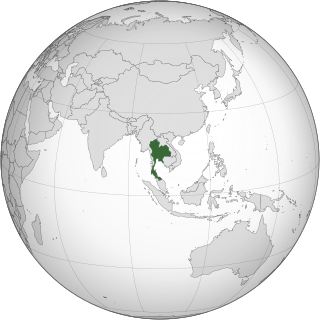
In Thailand, cannabis, known by the name Ganja has recently had new laws passed through. Cannabis that has less than 0.2% THC, referred to as industrial hemp in USA, was legalised on 9 June 2022. Medicinal cannabis, with no THC restrictions, was made legal in 2018 but required patients to obtain a prescription from a medical practitioner. Recreational cannabis is still illegal according to Thai law.
References
- ↑ "About us". Health Products Regulatory Authority. Retrieved 13 January 2019.
- ↑ "The Irish Times". www.irishtimes.com.
- ↑ "Health Products Regulatory Authority statement on drugs recall". Irish Times. 5 July 2018. Retrieved 13 January 2019.
- ↑ "Cannabis Regulation in Ireland". Lexology. 3 December 2018. Retrieved 13 January 2019.
- ↑ "Skincare copies: stock-up or avoid?". Image. 20 December 2018. Retrieved 13 January 2019.
- ↑ "Steroids and erectile dysfunction pills: One million doses of illegal medication seized last year". The Journal. 17 April 2018. Retrieved 13 January 2019.