Related Research Articles

Hepatitis is inflammation of the liver tissue. Some people or animals with hepatitis have no symptoms, whereas others develop yellow discoloration of the skin and whites of the eyes (jaundice), poor appetite, vomiting, tiredness, abdominal pain, and diarrhea. Hepatitis is acute if it resolves within six months, and chronic if it lasts longer than six months. Acute hepatitis can resolve on its own, progress to chronic hepatitis, or (rarely) result in acute liver failure. Chronic hepatitis may progress to scarring of the liver (cirrhosis), liver failure, and liver cancer.

Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) that primarily affects the liver; it is a type of viral hepatitis. During the initial infection period, people often have mild or no symptoms. Early symptoms can include fever, dark urine, abdominal pain, and yellow tinged skin. The virus persists in the liver, becoming chronic, in about 70% of those initially infected. Early on, chronic infection typically has no symptoms. Over many years however, it often leads to liver disease and occasionally cirrhosis. In some cases, those with cirrhosis will develop serious complications such as liver failure, liver cancer, or dilated blood vessels in the esophagus and stomach.
Hepatitis D is a type of viral hepatitis caused by the hepatitis delta virus (HDV). HDV is one of five known hepatitis viruses: A, B, C, D, and E. HDV is considered to be a satellite because it can propagate only in the presence of the hepatitis B virus (HBV). Transmission of HDV can occur either via simultaneous infection with HBV (coinfection) or superimposed on chronic hepatitis B or hepatitis B carrier state (superinfection).

Alcoholic liver disease (ALD), also called alcohol-related liver disease (ARLD), is a term that encompasses the liver manifestations of alcohol overconsumption, including fatty liver, alcoholic hepatitis, and chronic hepatitis with liver fibrosis or cirrhosis.

Hepatitis E is inflammation of the liver caused by infection with the hepatitis E virus (HEV); it is a type of viral hepatitis. Hepatitis E has mainly a fecal-oral transmission route that is similar to hepatitis A, although the viruses are unrelated. In retrospect, the earliest known epidemic of hepatitis E occurred in 1955 in New Delhi, but the virus was not isolated until 1983 by Russian scientists investigating an outbreak in Afghanistan. HEV is a positive-sense, single-stranded, nonenveloped, RNA icosahedral virus and one of five known human hepatitis viruses: A, B, C, D, and E.
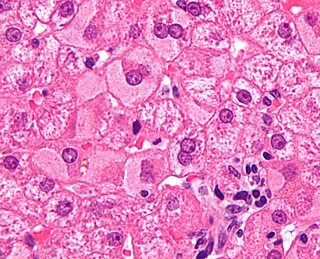
Viral hepatitis is liver inflammation due to a viral infection. It may present in acute form as a recent infection with relatively rapid onset, or in chronic form, typically progressing from a long-lasting asymptomatic condition up to a decompensated hepatic disease and hepatocellular carcinoma (HCC).

Autoimmune hepatitis, formerly known as lupoid hepatitis, plasma cell hepatitis, or autoimmune chronic active hepatitis, is a chronic, autoimmune disease of the liver that occurs when the body's immune system attacks liver cells, causing the liver to be inflamed. Common initial symptoms may include fatigue, nausea, muscle aches, or weight loss or signs of acute liver inflammation including fever, jaundice, and right upper quadrant abdominal pain. Individuals with autoimmune hepatitis often have no initial symptoms and the disease may be detected by abnormal liver function tests and increased protein levels during routine bloodwork or the observation of an abnormal-looking liver during abdominal surgery.
GB virus C (GBV-C), formerly known as hepatitis G virus (HGV) and also known as human pegivirus – HPgV is a virus in the family Flaviviridae and a member of the Pegivirus, is known to infect humans, but is not known to cause human disease. Reportedly, HIV patients coinfected with GBV-C can survive longer than those without GBV-C, but the patients may be different in other ways. Research is active into the virus' effects on the immune system in patients coinfected with GBV-C and HIV.
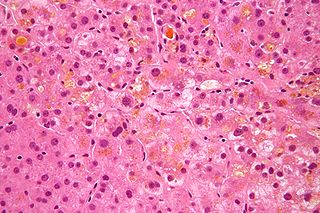
Cholestasis is a condition where the flow of bile from the liver to the duodenum is impaired. The two basic distinctions are:
The King's College Criteria or the King's College Hospital criteria were devised in 1989 to determine if there were any early indices of poor prognosis in patients with acute liver failure. Acute liver failure is defined as the onset of encephalopathy or coagulopathy within 26 weeks of a patient diagnosed with liver disease. Patients with hepatitis B acquired at birth, Wilson's disease and autoimmune hepatitis are included if their disease was identified within the past 26 weeks. These patients are very ill, and have a very high risk of dying of their illness without adequate treatment which may include liver transplantation. It is important that physicians find ways of identifying patients with acute liver failure early in their course who will do poorly, and may require liver transplantation. The King's College Criteria have consistently shown excellent operating characteristics for determining prognosis in these patients. As liver transplantation becomes a more accessible option for patients with acute liver failure, the King's College Criteria serve a role in determining which patients may require transplantation.
In medicine, the presence of elevated transaminases, commonly the transaminases alanine transaminase (ALT) and aspartate transaminase (AST), may be an indicator of liver dysfunction. Other terms include transaminasemia, transaminitis, and elevatedliver enzymes. Normal ranges for both ALT and AST vary by gender, age, and geography and are roughly 8-40 U/L. Mild transaminesemia refers to levels up to 250 U/L. Drug-induced increases such as that found with the use of anti-tuberculosis agents such as isoniazid are limited typically to below 100 U/L for either ALT or AST. Muscle sources of the enzymes, such as intense exercise, are unrelated to liver function and can markedly increase AST and ALT. Cirrhosis of the liver or fulminant liver failure secondary to hepatitis commonly reach values for both ALT and AST in the >1000 U/L range; however, many people with liver disease have normal transaminases. Elevated transaminases that persist less than six months are termed "acute" in nature, and those values that persist for six months or more are termed "chronic" in nature.

Hepatitis B is an infectious disease caused by the Hepatitis B virus (HBV) that affects the liver; it is a type of viral hepatitis. It can cause both acute and chronic infection.

E1 is one of two subunits of the envelope glycoprotein found in the hepatitis C virus. The other subunit is E2. This protein is a type 1 transmembrane protein with a highly glycosylated N-terminal ectodomain and a C-terminal hydrophobic anchor. After being synthesized the E1 glycoproteins associates with the E2 glycoprotein as a noncovalent heterodimer.

E2 is a viral structural protein found in the hepatitis C virus. It is present on the viral envelope and functions as a host receptor binding protein, mediating entry into host cells. It is a key target for the design of entry inhibitors and vaccine immunogens.

Hepatitis B virus (HBV) is a partially double-stranded DNA virus, a species of the genus Orthohepadnavirus and a member of the Hepadnaviridae family of viruses. This virus causes the disease hepatitis B.
FibroTest, known as FibroSure in the US, is a biomarker test that uses the results of six blood serum tests to generate a score that is correlated with the degree of liver damage in people with a variety of liver diseases. FibroTest has the same prognostic value as a liver biopsy. FibroSure uses quantitative results of five serum biochemical markers, α2-macroglobulin, haptoglobin, apolipoprotein A1, bilirubin, gamma glutamyl transpeptidase (GGT), with a patient’s age and gender to generate a measure of fibrosis and necroinflammatory activity in the liver.
Stuart C. Ray is an American physician. He is Vice Chair of Medicine for Data Integrity and Analytics, Associate Director of the Infectious Diseases Fellowship Training Program at the Johns Hopkins School of Medicine, and a Professor in the Department of Medicine, Division of Infectious Diseases. Ray also holds appointments in Viral Oncology and the Division of Health Sciences Informatics. He is affiliated with the Institute for Computational Medicine at Johns Hopkins and is licensed to practice medicine in Maryland.
Tetrabamate is a combination drug formulation of febarbamate, difebarbamate, and phenobarbital which was marketed in France and Spain and was used to treat anxiety and alcohol withdrawal-associated muscle tremors, agitation, and depression. It was largely, but not completely discontinued on April 4, 1997, after over 30 years of use due to reports of hepatitis and acute liver failure. The decision to restrict the use of the drug had been long-awaited.
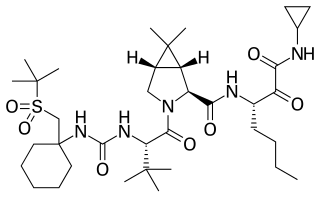
Narlaprevir, is an inhibitor of NS3/4A serine protease, intended for the treatment of chronic hepatitis C caused by genotype 1 virus in combination with other antiviral drugs.
The term Direct-acting antivirals (DAA) has long been associated with the combination of antiviral drugs used to treat hepatitis C infections. These are the more effective than older treatments such as ribavirin and interferon. The DAA drugs against hepatitis C are taken orally, as tablets, for 8 to 12 weeks. The treatment depends on the type or types (genotypes) of hepatitis C virus that are causing the infection. Both during and at the end of treatment, blood tests are used to monitor the effectiveness of the treatment and subsequent cure.
References
- ↑ "Non-A-E hepatitis". Genetic and Rare Diseases Information Center. U.S. Department of Health & Human Services. Retrieved March 26, 2019.
- ↑ Roberts, Sean. "Non-A-E hepatitis". NORD (National Organization for Rare Disorders). Retrieved 2022-08-12.