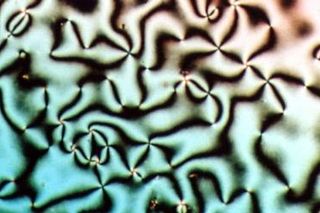
Liquid crystal (LC) is a state of matter that has properties between those of conventional liquids and those of solid crystals. For instance, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many different types of liquid-crystal phases, which can be distinguished by their different optical properties. The contrasting areas in the textures correspond to domains where the liquid-crystal molecules are oriented in different directions. Within a domain, however, the molecules are well ordered. LC materials may not always be in a liquid-crystal state of matter.

The lipid bilayer is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many viruses are made of a lipid bilayer, as are the nuclear membrane surrounding the cell nucleus, and membranes of the membrane-bound organelles in the cell. The lipid bilayer is the barrier that keeps ions, proteins and other molecules where they are needed and prevents them from diffusing into areas where they should not be. Lipid bilayers are ideally suited to this role, even though they are only a few nanometers in width, because they are impermeable to most water-soluble (hydrophilic) molecules. Bilayers are particularly impermeable to ions, which allows cells to regulate salt concentrations and pH by transporting ions across their membranes using proteins called ion pumps.
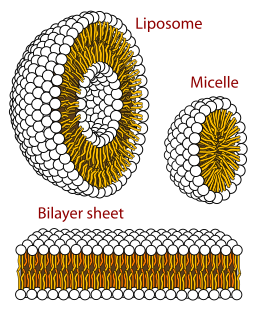
A micelle or micella is an aggregate of surfactant phospholipid molecules dispersed in a liquid, forming a colloidal suspension. A typical micelle in water forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre.

An amphiphile is a chemical compound possessing both hydrophilic and lipophilic (fat-loving) properties. Such a compound is called amphiphilic or amphipathic. Common amphiphilic substances are soaps, detergents, and lipoproteins. The phospholipid amphiphiles are the major structural component of cell membranes.
In microscopy, negative staining is an established method, often used in diagnostic microscopy, for contrasting a thin specimen with an optically opaque fluid. In this technique, the background is stained, leaving the actual specimen untouched, and thus visible. This contrasts with positive staining, in which the actual specimen is stained.
A lamella in biology refers to a thin layer, membrane or plate of tissue. This is a very broad definition, and can refer to many different structures. Any thin layer of organic tissue can be called a lamella and there is a wide array of functions an individual layer can serve. For example, an intercellular lipid lamella is formed when lamellar disks fuse to form a lamellar sheet. It is believed that these disks are formed from vesicles, giving the lamellar sheet a lipid bilayer that plays a role in water diffusion.

In cell biology, lamellar bodies are secretory organelles found in type II alveolar cells in the lungs, and in keratinocytes in the skin. They are oblong structures, appearing about 300-400 nm in width and 100-150 nm in length in transmission electron microscopy images. Lamellar bodies in the alveoli of the lungs fuse with the cell membrane and release pulmonary surfactant into the extracellular space.
In biology, membrane fluidity refers to the viscosity of the lipid bilayer of a cell membrane or a synthetic lipid membrane. Lipid packing can influence the fluidity of the membrane. Viscosity of the membrane can affect the rotation and diffusion of proteins and other bio-molecules within the membrane, there-by affecting the functions of these things.
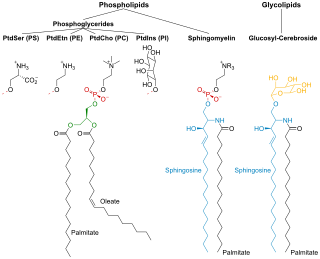
Membrane lipids are a group of compounds which form the double-layered surface of all cells. The three major classes of membrane lipids are phospholipids, glycolipids, and cholesterol. Lipids are amphiphilic: they have one end that is soluble in water ('polar') and an ending that is soluble in fat ('nonpolar'). By forming a double layer with the polar ends pointing outwards and the nonpolar ends pointing inwards membrane lipids can form a 'lipid bilayer' which keeps the watery interior of the cell separate from the watery exterior. The arrangements of lipids and various proteins, acting as receptors and channel pores in the membrane, control the entry and exit of other molecules and ions as part of the cell's metabolism. In order to perform physiological functions, membrane proteins are facilitated to rotate and diffuse laterally in two dimensional expanse of lipid bilayer by the presence of a shell of lipids closely attached to protein surface, called annular lipid shell.

Polymorphism in biophysics is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as "phases". This can be in the form of sphere of lipid molecules (micelles), pairs of layers that face one another, a tubular arrangement (hexagonal), or various cubic phases. More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases.
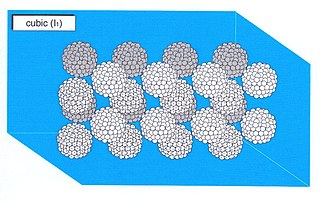
A micellar cubic phase is a lyotropic liquid crystal phase formed when the concentration of micelles dispersed in a solvent (usually water) is sufficiently high that they are forced to pack into a structure having long-ranged positional (translational) order. For example, spherical micelles a subic packing of a body-centred cubic lattice. Normal topology micellar cubic phases, denoted by the symbol I1, are the first lyotropic liquid crystalline phases that are formed by type I amphiphiles. The amphiphiles' hydrocarbon tails are contained on the inside of the micelle and hence the polar-apolar interface of the aggregates has a positive mean curvature, by definition (it curves away from the polar phase). Inverse topology micellar cubic phases (such as the Fd3m phase) are observed for some type II amphiphiles at very high amphiphile concentrations. These aggregates, in which water is the minority phase, have a polar-apolar interface with a negative mean curvature. The structures of the normal topology micellar cubic phases that are formed by some types of amphiphiles (e.g. the oligoethyleneoxide monoalkyl ether series of non-ionic surfactants are the subject of debate. Micellar cubic phases are isotropic phases, but are distinguished from micellar solutions by their very high viscosity. When thin film samples of micellar cubic phases are viewed under a polarising microscope they appear dark and featureless. Small air bubbles trapped in these preparations tend to appear highly distorted and occasionally have faceted surfaces.

A liquid crystalline mesophase is called lyotropic if formed by dissolving an amphiphilic mesogen in a suitable solvent, under appropriate conditions of concentration, temperature and pressure. A mixture of soap and water is an everyday example of a lyotropic liquid crystal.

An emulsion dispersion is thermoplastics or elastomers suspended in a liquid state by means of emulsifiers.
Lipid bilayer characterization is the use of various optical, chemical and physical probing methods to study the properties of lipid bilayers. Many of these techniques are elaborate and require expensive equipment because the fundamental nature of the lipid bilayer makes it a very difficult structure to study. An individual bilayer, since it is only a few nanometers thick, is invisible in traditional light microscopy. The bilayer is also a relatively fragile structure since it is held together entirely by non-covalent bonds and is irreversibly destroyed if removed from water. In spite of these limitations dozens of techniques have been developed over the last seventy years to allow investigations of the structure and function of bilayers. The first general approach was to utilize non-destructive in situ measurements such as x-ray diffraction and electrical resistance which measured bilayer properties but did not actually image the bilayer. Later, protocols were developed to modify the bilayer and allow its direct visualization at first in the electron microscope and, more recently, with fluorescence microscopy. Over the past two decades, a new generation of characterization tools including AFM has allowed the direct probing and imaging of membranes in situ with little to no chemical or physical modification. More recently, dual polarisation interferometry has been used to measure the optical birefringence of lipid bilayers to characterise order and disruption associated with interactions or environmental effects.
One property of a lipid bilayer is the relative mobility (fluidity) of the individual lipid molecules and how this mobility changes with temperature. This response is known as the phase behavior of the bilayer. Broadly, at a given temperature a lipid bilayer can exist in either a liquid or a solid phase. The solid phase is commonly referred to as a “gel” phase. All lipids have a characteristic temperature at which they undergo a transition (melt) from the gel to liquid phase. In both phases the lipid molecules are constrained to the two dimensional plane of the membrane, but in liquid phase bilayers the molecules diffuse freely within this plane. Thus, in a liquid bilayer a given lipid will rapidly exchange locations with its neighbor millions of times a second and will, through the process of a random walk, migrate over long distances.

The presence of ethanol can lead to the formations of non-lamellar phases also known as non-bilayer phases. Ethanol has been recognized as being an excellent solvent in an aqueous solution for inducing non-lamellar phases in phospholipids. The formation of non-lamellar phases in phospholipids is not completely understood, but it is significant that this amphiphilic molecule is capable of doing so. The formation of non-lamellar phases is significant in biomedical studies which include drug delivery, the transport of polar and non-polar ions using solvents capable of penetrating the biomembrane, increasing the elasticity of the biomembrane when it is being disrupted by unwanted substances and functioning as a channel or transporter of biomaterial.

Peptide amphiphiles (PAs) are peptide-based molecules that self-assemble into supramolecular nanostructures including; spherical micelles, twisted ribbons, and high-aspect-ratio nanofibers. A peptide amphiphile typically comprises a hydrophilic peptide sequence attached to a lipid tail, i.e. a hydrophobic alkyl chain with 10 to 16 carbons. Therefore, they can be considered a type of lipopeptide. A special type of PA, is constituted by alternating charged and neutral residues, in a repeated pattern, such as RADA16-I. The PAs were developed in the 1990s and the early 2000s and could be used in various medical areas including: nanocarriers, nanodrugs, and imaging agents. However, perhaps their main potential is in regenerative medicine to culture and deliver cells and growth factors.
For colloidal chemistry, the surfactant’s critical micelle concentration (CMC) plays a factor in Gibbs free energy of micellization. The exact concentration of the surfactants that yield the aggregates being thermodynamically soluble is the CMC. The Krafft temperature determines the solubility of the surfactants which in turn is the temperature that CMC is achieved. There are many parameters that affect the CMC. The interaction between the hydrophilic heads and the hydrophobic tails play a part, as well as the concentration of salt within the solution and surfactants.

Lamellar phase refers generally to packing of polar-headed long chain nonpolar-tail molecules in an environment of bulk polar liquid, as sheets of bilayers separated by bulk liquid. In biophysics, polar lipids pack as a liquid crystalline bilayer, with hydrophobic fatty acyl long chains directed inwardly and polar headgroups of lipids aligned on the outside in contact with water, as a 2-dimensional flat sheet surface. Under transmission electron microscope (TEM), after staining with polar headgroup reactive chemical osmium tetroxide, lamellar lipid phase appears as two thin parallel dark staining lines/sheets, constituted by aligned polar headgroups of lipids. 'Sandwiched' between these two parallel lines, there exists one thicker line/sheet of non-staining closely packed layer of long lipid fatty acyl chains. This TEM-appearance became famous as Robertson's unit membrane - the basis of all biological membranes, and structure of lipid bilayer in unilamellar liposomes. In multilamellar liposomes, many such lipid bilayer sheets are layered concentrically with water layers in between.
Before the emergence of electron microscopy in the 1950s, scientists did not know the structure of a cell membrane or what its components were; biologists and other researchers used indirect evidence to identify membranes before they could actually be visualized. Specifically, it was through the models of Overton, Langmuir, Gorter and Grendel, and Davson and Danielli, that it was deduced that membranes have lipids, proteins, and a bilayer. The advent of the electron microscope, the findings of J. David Robertson, the proposal of Singer and Nicolson, and additional work of Unwin and Henderson all contributed to the development of the modern membrane model. However, understanding of past membrane models elucidates present-day perception of membrane characteristics. Following intense experimental research, the membrane models of the preceding century gave way to the fluid mosaic model that is accepted today.















