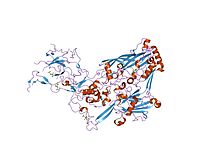
Integrins are transmembrane receptors that facilitate cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface.

Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can grow as long as 50 micrometres and are highly dynamic. The outer diameter of a microtubule is between 23 and 27 nm while the inner diameter is between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

Ribosomes are macromolecular machines, found within all living cells, that perform biological protein synthesis. Ribosomes link amino acids together in the order specified by the codons of messenger RNA (mRNA) molecules to form polypeptide chains. Ribosomes consist of two major components: the small and large ribosomal subunits. Each subunit consists of one or more ribosomal RNA (rRNA) molecules and many ribosomal proteins ). The ribosomes and associated molecules are also known as the translational apparatus.

Aspartate carbamoyltransferase catalyzes the first step in the pyrimidine biosynthetic pathway.
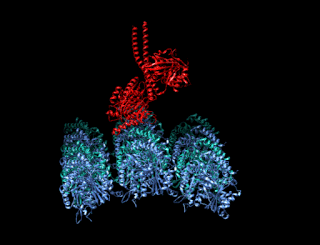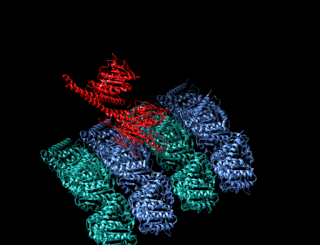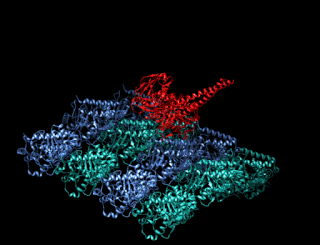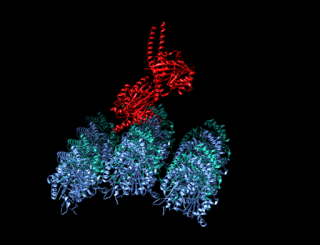
A kinesin is a protein belonging to a class of motor proteins found in eukaryotic cells.

Dynein is a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport, thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus end.
A coiled coil is a structural motif in proteins in which 2–7 alpha-helices are coiled together like the strands of a rope. Many coiled coil-type proteins are involved in important biological functions such as the regulation of gene expression, e.g. transcription factors. Notable examples are the oncoproteins c-Fos and c-jun, as well as the muscle protein tropomyosin.

Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers – specifically polypeptides – formed from sequences of amino acids, the monomers of the polymer. A single amino acid monomer may also be called a residue indicating a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reactions, in which the amino acids lose one water molecule per reaction in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their three-dimensional structure. This is the topic of the scientific field of structural biology, which employs techniques such as X-ray crystallography, NMR spectroscopy, cryo electron microscopy (cryo-EM) and dual polarisation interferometry to determine the structure of proteins.

Calnexin (CNX) is a 67kDa integral protein of the endoplasmic reticulum (ER). It consists of a large N-terminal calcium-binding lumenal domain, a single transmembrane helix and a short, acidic cytoplasmic tail.
The radial spoke is a multi-unit protein structure found in the axonemes of eukaryotic cilia and flagella. Although experiments have determined the importance of the radial spoke in the proper function of these organelles, its structure and mode of action remain poorly understood.

A DNA clamp, also known as a sliding clamp or β-clamp, is a protein complex that serves as a processivity-promoting factor in DNA replication. As a critical component of the DNA polymerase III holoenzyme, the clamp protein binds DNA polymerase and prevents this enzyme from dissociating from the template DNA strand. The clamp-polymerase protein–protein interactions are stronger and more specific than the direct interactions between the polymerase and the template DNA strand; because one of the rate-limiting steps in the DNA synthesis reaction is the association of the polymerase with the DNA template, the presence of the sliding clamp dramatically increases the number of nucleotides that the polymerase can add to the growing strand per association event. The presence of the DNA clamp can increase the rate of DNA synthesis up to 1,000-fold compared with a nonprocessive polymerase.

Molecular biophysics is a rapidly evolving interdisciplinary area of research that combines concepts in physics, chemistry, engineering, mathematics and biology. It seeks to understand biomolecular systems and explain biological function in terms of molecular structure, structural organization, and dynamic behaviour at various levels of complexity. This discipline covers topics such as the measurement of molecular forces, molecular associations, allosteric interactions, Brownian motion, and cable theory. Additional areas of study can be found on Outline of Biophysics. The discipline has required development of specialized equipment and procedures capable of imaging and manipulating minute living structures, as well as novel experimental approaches.

Dynactin subunit 1 is a protein that in humans is encoded by the DCTN1 gene.

Dynactin is a 23 subunit protein complex that acts as a co-factor for the microtubule motor cytoplasmic dynein-1. It is built around a short filament of actin related protein-1 (Arp1).
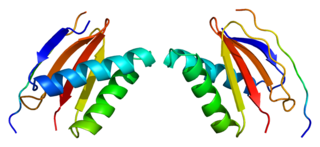
Dynein light chain 1, cytoplasmic is a protein that in humans is encoded by the DYNLL1 gene.

Dynactin subunit 2 is a protein that in humans is encoded by the DCTN2 gene

The G beta-gamma complex (Gβγ) is a tightly bound dimeric protein complex, composed of one Gβ and one Gγ subunit, and is a component of heterotrimeric G proteins. Heterotrimeric G proteins, also called guanosine nucleotide-binding proteins, consist of three subunits, called alpha, beta, and gamma subunits, or Gα, Gβ, and Gγ. When a G protein-coupled receptor (GPCR) is activated, Gα dissociates from Gβγ, allowing both subunits to perform their respective downstream signaling effects. One of the major functions of Gβγ is the inhibition of the Gα subunit.

In molecular biology the MYND-type zinc finger domain is a conserved protein domain. The MYND domain is present in a large group of proteins that includes RP-8 (PDCD2), Nervy, and predicted proteins from Drosophila, mammals, Caenorhabditis elegans, yeast, and plants. The MYND domain consists of a cluster of cysteine and histidine residues, arranged with an invariant spacing to form a potential zinc-binding motif. Mutating conserved cysteine residues in the DEAF-1 MYND domain does not abolish DNA binding, which suggests that the MYND domain might be involved in protein-protein interactions. Indeed, the MYND domain of ETO/MTG8 interacts directly with the N-CoR and SMRT co-repressors. Aberrant recruitment of co-repressor complexes and inappropriate transcriptional repression is believed to be a general mechanism of leukemogenesis caused by the t(8;21) translocations that fuse ETO with the acute myelogenous leukemia 1 (AML1) protein. ETO has been shown to be a co-repressor recruited by the promyelocytic leukemia zinc finger (PLZF) protein. A divergent MYND domain present in the adenovirus E1A binding protein BS69 was also shown to interact with N-CoR and mediate transcriptional repression. The current evidence suggests that the MYND motif in mammalian proteins constitutes a protein-protein interaction domain that functions as a co-repressor-recruiting interface.
Clathrin adaptor proteins, also known as adaptins, are vesicular transport adaptor proteins associated with clathrin. These proteins are synthesized in the ribosomes, processed in the endoplasmic reticulum and transported from the Golgi apparatus to the trans-Golgi network, and from there via small carrier vesicles to their final destination compartment. The association between adaptins and clathrin are important for vesicular cargo selection and transporting. Clathrin coats contain both clathrin and adaptor complexes that link clathrin to receptors in coated vesicles. Clathrin-associated protein complexes are believed to interact with the cytoplasmic tails of membrane proteins, leading to their selection and concentration. Therefore, adaptor proteins are responsible for the recruitment of cargo molecules into a growing clathrin-coated pits. The two major types of clathrin adaptor complexes are the heterotetrameric vesicular transport adaptor proteins (AP1-5), and the monomeric GGA adaptors. Adaptins are distantly related to the other main type of vesicular transport proteins, the coatomer subunits, sharing between 16% and 26% of their amino acid sequence.

Andrew P. Carter is a British structural biologist who works at the Medical Research Council (MRC) Laboratory of Molecular Biology (LMB) in Cambridge, UK. He is known for his work on the microtubule motor dynein.