
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active chemical substance is the same, the medical profile of generics is equivalent in performance compared to their performance at the time when they were patented drugs. A generic drug has the same active pharmaceutical ingredient (API) as the original, but it may differ in some characteristics such as the manufacturing process, formulation, excipients, color, taste, and packaging.
Ranbaxy Laboratories Limited was an Indian multinational pharmaceutical company that was incorporated in India in 1961 and remained an entity until 2014. The company went public in 1973. Ownership of Ranbaxy changed twice over the course of its history.
Dr. Reddy's Laboratories is an Indian multinational pharmaceutical company based in Hyderabad. The company was founded by Kallam Anji Reddy, who previously worked in the mentor institute Indian Drugs and Pharmaceuticals Limited. Dr. Reddy manufactures and markets a wide range of pharmaceuticals in India and overseas. The company produces over 190 medications, 60 active pharmaceutical ingredients (APIs) for drug manufacture, diagnostic kits, critical care, and biotechnology.
The pharmaceutical industry is one of the leading industries in the People's Republic of China, covering synthetic chemicals and drugs, prepared Chinese medicines, medical devices, apparatus and instruments, hygiene materials, packing materials, and pharmaceutical machinery. China has the second-largest pharmaceutical market in the world as of 2017 which is worth US$110 billion. China accounts for 20% of the world's population but only a small fraction of the global drug market. China's changing health-care environment is designed to extend basic health insurance to a larger portion of the population and give individuals greater access to products and services. Following the period of change, the pharmaceutical industry is expected to continue its expansion.

Johnson & Johnson Innovative Medicine is a Belgian pharmaceutical company headquartered in Beerse, Belgium, and wholly-owned by Johnson & Johnson. It was founded in 1953 by Paul Janssen.
The pharmaceutical industry in India was valued at an estimated US$42 billion in 2021 and is estimated to reach $130 billion by 2030. India is the world's largest provider of generic medicines by volume, with a 20% share of total global pharmaceutical exports. It is also the largest vaccine supplier in the world by volume, accounting for more than 60% of all vaccines manufactured in the world. Indian pharmaceutical products are exported to various regulated markets including the US, UK, European Union and Canada.
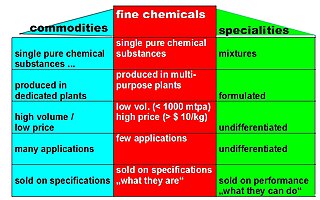
In chemistry, fine chemicals are complex, single, pure chemical substances, produced in limited quantities in multipurpose plants by multistep batch chemical or biotechnological processes. They are described by exacting specifications, used for further processing within the chemical industry and sold for more than $10/kg. The class of fine chemicals is subdivided either on the basis of the added value, or the type of business transaction, namely standard or exclusive products.

Apotex Inc. is a Canadian pharmaceutical corporation. Founded in 1974 by Barry Sherman, the company is the largest producer of generic drugs in Canada, with annual sales exceeding CA$2.5 billion. By 2023, Apotex employed close to 8,000 people as Canada's largest drug manufacturer, with over 300 products selling in over 115 countries. Apotex manufactures and distributes generic medications for a range of diseases and health conditions that include cancer, diabetes, high cholesterol, glaucoma, infections and blood pressure.
RespireRx Pharmaceuticals Inc. is a pharmaceutical company based in Glen Rock, New Jersey specializing in positive allosteric modulators of the AMPA receptor known as Ampakines.
The National Institute for Pharmaceutical Technology and Education (NIPTE) is a non-profit scientific and research and development organization that was established in 2005 and incorporated in June 2007 in the State of Indiana. Its offices are currently located in Minneapolis, Minnesota.
Emcure Pharmaceuticals Limited is an Indian multinational pharmaceutical company, headquartered in Pune. Emcure's product portfolio includes tablets, capsules and injectables. The company produces gynaecology, cardiovascular, oncology and blood therapeutic drugs, HIV antivirals and other anti-infectives, and vitamins and minerals.
Teva API is an international pharmaceutical company headquartered in Israel. Teva API is a stand-alone business unit of Teva Pharmaceutical Industries limited, the largest generic drug manufacturer in the world and one of the 15 largest pharmaceutical companies worldwide.

Amneal Pharmaceuticals, Inc. is an American publicly traded generics and specialty pharmaceutical company. The company is headquartered in Bridgewater, New Jersey.

Taro Pharmaceutical Industries is an Israeli research-based pharmaceutical manufacturer that was publicly listed in the New York Stock Exchange before it was acquired by Sun Pharma. The company has more than 180 of its own drugs sold all over the world, reaching the markets of over 25 countries. The company's products are mainly sold in the United States, Canada and Israel.

A deuterated drug is a small molecule medicinal product in which one or more of the hydrogen atoms in the drug molecule have been replaced by its heavier stable isotope deuterium. Because of the kinetic isotope effect, deuterium-containing drugs may have significantly lower rates of metabolism, and hence a longer half-life.
Joseph Fuisz is an American attorney, inventor, and entrepreneur of Slovenian descent. He works predominantly in the pharmaceutical industry as the founder of Fuisz Pharma LLC. As of October 2015, he is named on 32 medical patents, and over forty patents.
Alembic Pharmaceuticals Ltd. is an Indian multinational pharmaceutical company headquartered in Vadodara. It is involved in the manufacture of pharmaceutical products, pharmaceutical substances and intermediates. It is also termed to be a market leader in macrolides segment of anti-infective drugs in India.

Arven Pharmaceuticals is a Turkish pharmaceutical corporation headquartered in Istanbul established as a subsidiary of Toksöz Group in 2013. Arven's primary focus is development and production of high-technology inhaler and biotechnology products. The company is specialized on difficult to make products. Arven is the first Turkish company developing biosimilars for global markets, including the US and EU.
Relief Therapeutics is a Swiss biopharmaceutical company based in Geneva. The company focuses on developing drugs for serious diseases with few or no existing treatment options. Its lead compound, RLF-100, is a synthetic form of a natural peptide that protects the lung. The company was incorporated as Relief Therapeutics Holdings AG (RFLB.S) and listed on the SIX Swiss Exchange in 2016.

ApiJect Systems Corporation is an American company founded in 2018 by Marc Koska and based in Stamford, Connecticut that produces pre-filled single use plastic injectors. ApiJect works with pharmaceutical and biotech companies to fill their injectable drug products into single-dose prefilled injectors. The company claimed to have the capacity to manufacture pre-filled COVID-19 vaccine syringes by the end of 2020.









