
The Papanicolaou test is a method of cervical screening used to detect potentially precancerous and cancerous processes in the cervix or colon. Abnormal findings are often followed up by more sensitive diagnostic procedures and, if warranted, interventions that aim to prevent progression to cervical cancer. The test was independently invented in the 1920s by the Greek physician Georgios Papanikolaou and named after him. A simplified version of the test was introduced by the Canadian obstetrician Anna Marion Hilliard in 1957.

Cervical cancer is a cancer arising from the cervix. It is due to the abnormal growth of cells that have the ability to invade or spread to other parts of the body. Early on, typically no symptoms are seen. Later symptoms may include abnormal vaginal bleeding, pelvic pain or pain during sexual intercourse. While bleeding after sex may not be serious, it may also indicate the presence of cervical cancer.
Human papillomavirus infection is caused by a DNA virus from the Papillomaviridae family. Many HPV infections cause no symptoms and 90% resolve spontaneously within two years. In some cases, an HPV infection persists and results in either warts or precancerous lesions. These lesions, depending on the site affected, increase the risk of cancer of the cervix, vulva, vagina, penis, anus, mouth, tonsils, or throat. Nearly all cervical cancer is due to HPV and two strains – HPV16 and HPV18 – which account for 70% of cases. HPV16 is responsible for almost 90% of HPV-positive oropharyngeal cancers. Between 60% and 90% of the other cancers listed above are also linked to HPV. HPV6 and HPV11 are common causes of genital warts and laryngeal papillomatosis.
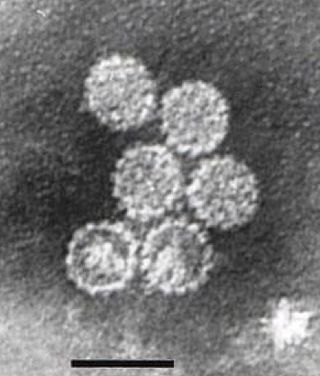
Papillomaviridae is a family of non-enveloped DNA viruses whose members are known as papillomaviruses. Several hundred species of papillomaviruses, traditionally referred to as "types", have been identified infecting all carefully inspected mammals, but also other vertebrates such as birds, snakes, turtles and fish. Infection by most papillomavirus types, depending on the type, is either asymptomatic or causes small benign tumors, known as papillomas or warts. Papillomas caused by some types, however, such as human papillomaviruses 16 and 18, carry a risk of becoming cancerous.

Anal cancer is a cancer which arises from the anus, the distal opening of the gastrointestinal tract. Symptoms may include bleeding from the anus or a lump near the anus. Other symptoms may include pain, itchiness, or discharge from the anus. A change in bowel movements may also occur.

Head and neck cancer develops from tissues in the lip and oral cavity (mouth), larynx (throat), salivary glands, nose, sinuses, or skin of the face. The most common types of head and neck cancer occur in the lips, mouth, and larynx. Symptoms predominantly include a sore that does not heal or a change in the voice. In those with advanced disease, there may be unusual bleeding, facial pain, numbness or swelling, and visible lumps on the outside of the neck or oral cavity. Given the location of these cancers, it is possible for an afflicted individual to experience difficulty in breathing.

A precancerous condition is a condition, tumor or lesion involving abnormal cells which are associated with an increased risk of developing into cancer. Clinically, precancerous conditions encompass a variety of abnormal tissues with an increased risk of developing into cancer. Some of the most common precancerous conditions include certain colon polyps, which can progress into colon cancer, monoclonal gammopathy of undetermined significance, which can progress into multiple myeloma or myelodysplastic syndrome. and cervical dysplasia, which can progress into cervical cancer. Bronchial premalignant lesions can progress to squamous cell carcinoma of the lung.

Cervical intraepithelial neoplasia (CIN), also known as cervical dysplasia, is the abnormal growth of cells on the surface of the cervix that could potentially lead to cervical cancer. More specifically, CIN refers to the potentially precancerous transformation of cells of the cervix.

Human papillomavirus (HPV) vaccines are vaccines that prevent infection by certain types of human papillomavirus (HPV). Available HPV vaccines protect against either two, four, or nine types of HPV. All HPV vaccines protect against at least HPV types 16 and 18, which cause the greatest risk of cervical cancer. It is estimated that HPV vaccines may prevent 70% of cervical cancer, 80% of anal cancer, 60% of vaginal cancer, 40% of vulvar cancer, and show more than 90% efficacy in preventing HPV-positive oropharyngeal cancers. They additionally prevent some genital warts, with the quadrivalent and nonavalent vaccines that protect against HPV types HPV-6 and HPV-11 providing greater protection.

Ian Hector Frazer is a Scottish-born Australian immunologist, the founding CEO and Director of Research of the Translational Research Institute (Australia). Frazer and Jian Zhou developed and patented the basic technology behind the HPV vaccine against cervical cancer at the University of Queensland. Researchers at the National Cancer Institute, Georgetown University, and University of Rochester also contributed to the further development of the cervical cancer vaccine in parallel.

A koilocyte is a squamous epithelial cell that has undergone a number of structural changes, which occur as a result of infection of the cell by human papillomavirus (HPV). Identification of these cells by pathologists can be useful in diagnosing various HPV-associated lesions.
Advaxis Inc. is an American company devoted to the discovery, development and commercialization of immunotherapies based on a technology platform which uses engineered Listeria monocytogenes. The company is headquartered in Princeton, New Jersey and was incorporated in Delaware in 2006.

Gardasil is an HPV vaccine for use in the prevention of certain strains of human papillomavirus (HPV). It was developed by Merck & Co. High-risk human papilloma virus (hr-HPV) genital infection is the most common sexually transmitted infection among women. The HPV strains that Gardasil protects against are sexually transmitted, specifically HPV types 6, 11, 16 and 18. HPV types 16 and 18 cause an estimated 70% of cervical cancers, and are responsible for most HPV-induced anal, vulvar, vaginal, and penile cancer cases. HPV types 6 and 11 cause an estimated 90% of genital warts cases. HPV type 16 is responsible for almost 90% of HPV-positive oropharyngeal cancers, and the prevalence is higher in males than females. Though Gardasil does not treat existing infection, vaccination is still recommended for HPV-positive individuals, as it may protect against one or more different strains of the disease.

Bovine papillomaviruses (BPV) are a paraphyletic group of DNA viruses of the subfamily Firstpapillomavirinae of Papillomaviridae that are common in cattle. All BPVs have a circular double-stranded DNA genome. Infection causes warts of the skin and alimentary tract, and more rarely cancers of the alimentary tract and urinary bladder. They are also thought to cause the skin tumour equine sarcoid in horses and donkeys.
Cervarix is a vaccine against certain types of cancer-causing human papillomavirus (HPV).

Nventa Biopharmaceuticals Corporation was a Canadian-incorporated biopharmaceutical company headquartered in San Diego, California developing therapeutics for the treatment of viral infections and cancer, focusing on diseases caused by human papillomavirus (HPV). Nventa is currently the only company applying heat shock protein (Hsp) technology to target the over 20 million Americans already infected with HPV. Previously headquartered in Victoria, British Columbia, Canada, the company’s common stock traded on the Toronto Stock Exchange under the symbol: NVN.
An anal Pap smear is the anal counterpart of the cervical Pap smear. It is used for the early detection of anal cancer. Some types of human papillomavirus (HPV) can cause anal cancer. Other HPV types cause anogenital warts. Cigarette smokers, men who have sex with men, individuals with a history of immunosuppression and women with a history of cervical, vaginal and vulval cancer are at increased risk of getting anal cancer. Vaccination against HPV before initial sexual exposure can reduce the risk of anal cancer.

Oropharyngeal cancer, also known as oropharyngeal squamous cell carcinoma and tonsil cancer, is a disease in which abnormal cells with the potential to both grow locally and spread to other parts of the body are found in the oral cavity, in the tissue of the part of the throat (oropharynx) that includes the base of the tongue, the tonsils, the soft palate, and the walls of the pharynx.

Human papillomavirus-positive oropharyngeal cancer, is a cancer of the throat caused by the human papillomavirus type 16 virus (HPV16). In the past, cancer of the oropharynx (throat) was associated with the use of alcohol or tobacco or both, but the majority of cases are now associated with the HPV virus, acquired by having oral contact with the genitals of a person who has a genital HPV infection. Risk factors include having a large number of sexual partners, a history of oral-genital sex or anal–oral sex, having a female partner with a history of either an abnormal Pap smear or cervical dysplasia, having chronic periodontitis, and, among men, younger age at first intercourse and a history of genital warts. HPV-positive OPC is considered a separate disease from HPV-negative oropharyngeal cancer.

Cervical cancer screening is a medical screening test designed to identify risk of cervical cancer. Cervical screening may involve looking for viral DNA, and/or to identify abnormal, potentially precancerous cells within the cervix as well as cells that have progressed to early stages of cervical cancer. One goal of cervical screening is to allow for intervention and treatment so abnormal lesions can be removed prior to progression to cancer. An additional goal is to decrease mortality from cervical cancer by identifying cancerous lesions in their early stages and providing treatment prior to progression to more invasive disease.















