
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose, and alginate. Other examples of biopolymers include natural rubbers, suberin and lignin, cutin and cutan, melanin, and polyhydroxyalkanoates (PHAs).
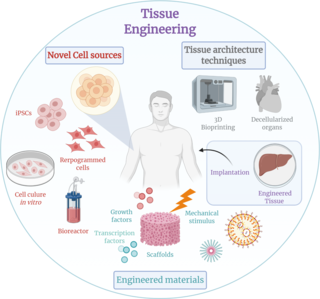
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose, but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance, it can be considered as a field of its own.

A hydrogel is a biphasic material, a mixture of porous and permeable solids and at least 10% of water or other interstitial fluid. The solid phase is a water insoluble three dimensional network of polymers, having absorbed a large amount of water or biological fluids. Hydrogels have several applications, especially in the biomedical area, such as in hydrogel dressing. Many hydrogels are synthetic, but some are derived from natural materials. The term "hydrogel" was coined in 1894.

Chitosan is a linear polysaccharide composed of randomly distributed β-(1→4)-linked D-glucosamine and N-acetyl-D-glucosamine. It is made by treating the chitin shells of shrimp and other crustaceans with an alkaline substance, such as sodium hydroxide.

Alginic acid, also called algin, is a naturally occurring, edible polysaccharide found in brown algae. It is hydrophilic and forms a viscous gum when hydrated. When the alginic acid binds with sodium and calcium ions, the resulting salts are known as alginates. Its colour ranges from white to yellowish-brown. It is sold in filamentous, granular, or powdered forms.
A dressing or compress is a piece of material such as a pad applied to a wound to promote healing and protect the wound from further harm. A dressing is designed to be in direct contact with the wound, as distinguished from a bandage, which is most often used to hold a dressing in place. Modern dressings are sterile.
Supramolecular polymers are a subset of polymers where the monomeric units are connected by reversible and highly directional secondary interactions–that is, non-covalent bonds. These non-covalent interactions include van der Waals interactions, hydrogen bonding, Coulomb or ionic interactions, π-π stacking, metal coordination, halogen bonding, chalcogen bonding, and host–guest interaction. Their behavior can be described by the theories of polymer physics) in dilute and concentrated solution, as well as in the bulk.
Thiolated polymers – designated thiomers – are functional polymers used in biotechnology product development with the intention to prolong mucosal drug residence time and to enhance absorption of drugs. The name thiomer was coined by Andreas Bernkop-Schnürch in 2000. Thiomers have thiol bearing side chains. Sulfhydryl ligands of low molecular mass are covalently bound to a polymeric backbone consisting of mainly biodegradable polymers, such as chitosan, hyaluronic acid, cellulose derivatives, pullulan, starch, gelatin, polyacrylates, cyclodextrins, or silicones.
A nanogel is a polymer-based, crosslinked hydrogel particle on the sub-micron scale. These complex networks of polymers present a unique opportunity in the field of drug delivery at the intersection of nanoparticles and hydrogel synthesis. Nanogels can be natural, synthetic, or a combination of the two and have a high degree of tunability in terms of their size, shape, surface functionalization, and degradation mechanisms. Given these inherent characteristics in addition to their biocompatibility and capacity to encapsulate small drugs and molecules, nanogels are a promising strategy to treat disease and dysfunction by serving as delivery vehicles capable of navigating across challenging physiological barriers within the body.
Nano-scaffolding or nanoscaffolding is a medical process used to regrow tissue and bone, including limbs and organs. The nano-scaffold is a three-dimensional structure composed of polymer fibers very small that are scaled from a Nanometer scale. Developed by the American military, the medical technology uses a microscopic apparatus made of fine polymer fibers called a scaffold. Damaged cells grip to the scaffold and begin to rebuild missing bone and tissue through tiny holes in the scaffold. As tissue grows, the scaffold is absorbed into the body and disappears completely.

Arginylglycylaspartic acid (RGD) is the most common peptide motif responsible for cell adhesion to the extracellular matrix (ECM), found in species ranging from Drosophila to humans. Cell adhesion proteins called integrins recognize and bind to this sequence, which is found within many matrix proteins, including fibronectin, fibrinogen, vitronectin, osteopontin, and several other adhesive extracellular matrix proteins. The discovery of RGD and elucidation of how RGD binds to integrins has led to the development of a number of drugs and diagnostics, while the peptide itself is used ubiquitously in bioengineering. Depending on the application and the integrin targeted, RGD can be chemically modified or replaced by a similar peptide which promotes cell adhesion.
Smart polymers, stimuli-responsive polymers or functional polymers are high-performance polymers that change according to the environment they are in.
Polymers with the ability to kill or inhibit the growth of microorganisms such as bacteria, fungi, or viruses are classified as antimicrobial agents. This class of polymers consists of natural polymers with inherent antimicrobial activity and polymers modified to exhibit antimicrobial activity. Polymers are generally nonvolatile, chemically stable, and can be chemically and physically modified to display desired characteristics and antimicrobial activity. Antimicrobial polymers are a prime candidate for use in the food industry to prevent bacterial contamination and in water sanitation to inhibit the growth of microorganisms in drinking water.
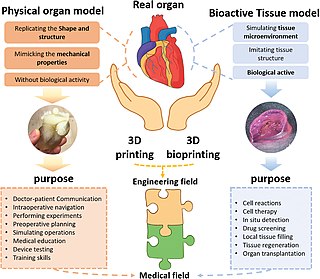
Three dimensional (3D) bioprinting is the use of 3D printing–like techniques to combine cells, growth factors, bio-inks, and biomaterials to fabricate functional structures that were traditionally used for tissue engineering applications but in recent times have seen increased interest in other applications such as biosensing, and environmental remediation. Generally, 3D bioprinting uses a layer-by-layer method to deposit materials known as bio-inks to create tissue-like structures that are later used in various medical and tissue engineering fields. 3D bioprinting covers a broad range of bioprinting techniques and biomaterials. Currently, bioprinting can be used to print tissue and organ models to help research drugs and potential treatments. Nonetheless, translation of bioprinted living cellular constructs into clinical application is met with several issues due to the complexity and cell number necessary to create functional organs. However, innovations span from bioprinting of extracellular matrix to mixing cells with hydrogels deposited layer by layer to produce the desired tissue. In addition, 3D bioprinting has begun to incorporate the printing of scaffolds which can be used to regenerate joints and ligaments. Apart from these, 3D bioprinting has recently been used in environmental remediation applications, including the fabrication of functional biofilms that host functional microorganisms that can facilitate pollutant removal.

Self-healing hydrogels are a specialized type of polymer hydrogel. A hydrogel is a macromolecular polymer gel constructed of a network of crosslinked polymer chains. Hydrogels are synthesized from hydrophilic monomers by either chain or step growth, along with a functional crosslinker to promote network formation. A net-like structure along with void imperfections enhance the hydrogel's ability to absorb large amounts of water via hydrogen bonding. As a result, hydrogels, self-healing alike, develop characteristic firm yet elastic mechanical properties. Self-healing refers to the spontaneous formation of new bonds when old bonds are broken within a material. The structure of the hydrogel along with electrostatic attraction forces drive new bond formation through reconstructive covalent dangling side chain or non-covalent hydrogen bonding. These flesh-like properties have motivated the research and development of self-healing hydrogels in fields such as reconstructive tissue engineering as scaffolding, as well as use in passive and preventive applications.
Electronic skin refers to flexible, stretchable and self-healing electronics that are able to mimic functionalities of human or animal skin. The broad class of materials often contain sensing abilities that are intended to reproduce the capabilities of human skin to respond to environmental factors such as changes in heat and pressure.
Nanocomposite hydrogels are nanomaterial-filled, hydrated, polymeric networks that exhibit higher elasticity and strength relative to traditionally made hydrogels. A range of natural and synthetic polymers are used to design nanocomposite network. By controlling the interactions between nanoparticles and polymer chains, a range of physical, chemical, and biological properties can be engineered. The combination of organic (polymer) and inorganic (clay) structure gives these hydrogels improved physical, chemical, electrical, biological, and swelling/de-swelling properties that cannot be achieved by either material alone. Inspired by flexible biological tissues, researchers incorporate carbon-based, polymeric, ceramic and/or metallic nanomaterials to give these hydrogels superior characteristics like optical properties and stimulus-sensitivity which can potentially be very helpful to medical and mechanical fields.
Artificial cartilage is a synthetic material made of hydrogels or polymers that aims to mimic the functional properties of natural cartilage in the human body. Tissue engineering principles are used in order to create a non-degradable and biocompatible material that can replace cartilage. While creating a useful synthetic cartilage material, certain challenges need to be overcome. First, cartilage is an avascular structure in the body and therefore does not repair itself. This creates issues in regeneration of the tissue. Synthetic cartilage also needs to be stably attached to its underlying surface i.e. the bone. Lastly, in the case of creating synthetic cartilage to be used in joint spaces, high mechanical strength under compression needs to be an intrinsic property of the material.
Bioprinting drug delivery is a method for producing drug delivery vehicles. It uses three-dimensional printing of biomaterials via additive manufacturing. Such vehicles are biocompatible, tissue-specific hydrogels or implantable devices. 3D bioprinting prints cells and biological molecules to form tissues, organs, or biological materials in a scaffold-free manner that mimics living human tissue. The technique allows targeted disease treatments with scalable and complex geometry.
Ultrasound-triggered drug delivery using stimuli-responsive hydrogels refers to the process of using ultrasound energy for inducing drug release from hydrogels that are sensitive to acoustic stimuli. This method of approach is one of many stimuli-responsive drug delivery-based systems that has gained traction in recent years due to its demonstration of localization and specificity of disease treatment. Although recent developments in this field highlight its potential in treating certain diseases such as COVID-19, there remain many major challenges that need to be addressed and overcome before more related biomedical applications are clinically translated into standard of care.








