Related Research Articles

The olfactory nerve, also known as the first cranial nerve, cranial nerve I, or simply CN I, is a cranial nerve that contains sensory nerve fibers relating to the sense of smell.

Olfactory receptors (ORs), also known as odorant receptors, are chemoreceptors expressed in the cell membranes of olfactory receptor neurons and are responsible for the detection of odorants which give rise to the sense of smell. Activated olfactory receptors trigger nerve impulses which transmit information about odor to the brain. In vertebrates, these receptors are members of the class A rhodopsin-like family of G protein-coupled receptors (GPCRs). The olfactory receptors form the largest multigene family in vertebrates consisting of around 400 genes in humans and 1400 genes in mice. In insects, olfactory receptors are members of an unrelated group of ligand-gated ion channels.

Androstenone (5α-androst-16-en-3-one) is a 16-androstene class steroidal pheromone. It is found in boar's saliva, celery cytoplasm, and truffle fungus. Androstenone was the first mammalian pheromone to be identified. It is found in high concentrations in the saliva of male pigs, and, when inhaled by a female pig that is in heat, results in the female assuming the mating stance. Androstenone is the active ingredient in 'Boarmate', a commercial product made by DuPont sold to pig farmers to test sows for timing of artificial insemination.

The norepinephrine transporter (NET), also known as noradrenaline transporter (NAT), is a protein that in humans is encoded by the solute carrier family 6 member 2 (SLC6A2) gene.
Trace amine-associated receptors (TAARs), sometimes referred to as trace amine receptors, are a class of G protein-coupled receptors that were discovered in 2001. TAAR1, the first of six functional human TAARs, has gained considerable interest in academic and proprietary pharmaceutical research due to its role as the endogenous receptor for the trace amines phenethylamine, tyramine, and tryptamine – metabolic derivatives of the amino acids phenylalanine, tyrosine and tryptophan, respectively – ephedrine, as well as the synthetic psychostimulants, amphetamine, methamphetamine and methylenedioxymethamphetamine. In 2004, it was shown that mammalian TAAR1 is also a receptor for thyronamines, decarboxylated and deiodinated relatives of thyroid hormones. TAAR2–TAAR9 function as olfactory receptors for volatile amine odorants in vertebrates.
In neuroanatomy, topographic map is the ordered projection of a sensory surface or an effector system to one or more structures of the central nervous system. Topographic maps can be found in all sensory systems and in many motor systems.

Dopamine receptor D5, also known as D1BR, is a protein that in humans is encoded by the DRD5 gene. It belongs to the D1-like receptor family along with the D1 receptor subtype.

Trace amine-associated receptor 2 (TAAR2), formerly known as G protein-coupled receptor 58 (GPR58), is a protein that in humans is encoded by the TAAR2 gene. TAAR2 is co-expressed with Gα proteins; however, as of February 2017, its signal transduction mechanisms have not been determined.

Olfactory receptor 51E2 is a protein that in humans is encoded by the OR51E2 gene.

Olfactory receptor 11H6 is a protein that in humans is encoded by the OR11H6 gene.

Olfactory receptor 7D4 is a protein that in humans is encoded by the OR7D4 gene.

Olfactory receptor 51E1 is a protein that in humans is encoded by the OR51E1 gene.

Olfactory receptor 5A1 is a protein that in humans is encoded by the OR5A1 gene.

Olfactory receptor 11H4 is a protein that in humans is encoded by the OR11H4 gene.

Olfactory receptor 2J3 is a protein that in humans is encoded by the OR2J3 gene.

The sense of smell, or olfaction, is the special sense through which smells are perceived. The sense of smell has many functions, including detecting desirable foods, hazards, and pheromones, and plays a role in taste.
Olfactory memory refers to the recollection of odors. Studies have found various characteristics of common memories of odor memory including persistence and high resistance to interference. Explicit memory is typically the form focused on in the studies of olfactory memory, though implicit forms of memory certainly supply distinct contributions to the understanding of odors and memories of them. Research has demonstrated that the changes to the olfactory bulb and main olfactory system following birth are extremely important and influential for maternal behavior. Mammalian olfactory cues play an important role in the coordination of the mother infant bond, and the following normal development of the offspring. Maternal breast odors are individually distinctive, and provide a basis for recognition of the mother by her offspring.
Odor molecules are detected by the olfactory receptors in the olfactory epithelium of the nasal cavity. Each receptor type is expressed within a subset of neurons, from which they directly connect to the olfactory bulb in the brain. Olfaction is essential for survival in most vertebrates; however, the degree to which an animal depends on smell is highly varied. Great variation exists in the number of OR genes among vertebrate species, as shown through bioinformatic analyses. This diversity exists by virtue of the wide-ranging environments that they inhabit. For instance, dolphins that are secondarily adapted to an aquatic niche possess a considerably smaller subset of genes than most mammals. OR gene repertoires have also evolved in relation to other senses, as higher primates with well-developed vision systems tend to have a smaller number of OR genes. As such, investigating the evolutionary changes of OR genes can provide useful information on how genomes respond to environmental changes. Differences in smell sensitivity are also dependent on the anatomy of the olfactory apparatus, such as the size of the olfactory bulb and epithelium.

Olfactory receptor family 1 subfamily E member 3 (gene/pseudogene) is a protein that in humans is encoded by the OR1E3 gene.
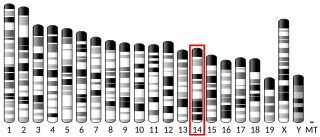
Olfactory receptor family 11 subfamily H member 7 (gene/pseudogene) is a protein that in humans is encoded by the OR11H7 gene.
References
- ↑ Walker, HK (1990). Clinical Methods: The History, Physical, and Laboratory Examinations. Boston: Butterworths. pp. Ch 59.
- ↑ Menashe, I; Abaffy, T; Hasin, Y; Goshen, S; Yahalom, V; Luetje, CW; Lancet, D (2007-10-30). "Genetic elucidation of human hyperosmia to isovaleric acid". PLOS Biology. 5 (11): e284. doi: 10.1371/journal.pbio.0050284 . PMC 2043052 . PMID 17973576.
- ↑ Keller, A; Zhuang, H; Chi, Q; Vosshall, LB; Matsunami, H (2007-09-27). "Genetic variation in a human odorant receptor alters odour perception". Nature. 449 (7161): 468–72. Bibcode:2007Natur.449..468K. doi:10.1038/nature06162. PMID 17873857. S2CID 4417235.
- ↑ Atianjoh, FE; Ladenheim, B; Krasnova, IN; Cadet, JL (2008-07-28). "Amphetamine causes dopamine depletion and cell death in the mouse olfactory bulb". European Journal of Pharmacology. 589 (1–3): 94–7. doi:10.1016/j.ejphar.2008.05.001. PMC 2536718 . PMID 18544452.
- 1 2 Sacks, Oliver (1985). The Man Who Mistook His Wife for a Hat . New York: Simon & Schuster. pp. 156–160. ISBN 978-0-684-85394-9.
- 1 2 3 4 Henkin, RI (1990-12-05). "Hyperosmia and depression following exposure to toxic vapors". JAMA: The Journal of the American Medical Association. 264 (21): 2803. doi:10.1001/jama.264.21.2803. PMID 2232068.
- ↑ Zargari, O (2006-12-10). "Methotrexate, hyperosmia, and migraine". Dermatology Online Journal. 12 (7): 28. doi:10.5070/D34PT9B52W. PMID 17459314. Abstract.