Related Research Articles
Sharpless asymmetric dihydroxylation is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol. The reaction has been applied to alkenes of virtually every substitution, often high enantioselectivities are realized, with the chiral outcome controlled by the choice of dihydroquinidine (DHQD) vs dihydroquinine (DHQ) as the ligand. Asymmetric dihydroxylation reactions are also highly site selective, providing products derived from reaction of the most electron-rich double bond in the substrate.

Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts."
The Nazarov cyclization reaction is a chemical reaction used in organic chemistry for the synthesis of cyclopentenones. The reaction is typically divided into classical and modern variants, depending on the reagents and substrates employed. It was originally discovered by Ivan Nikolaevich Nazarov (1906–1957) in 1941 while studying the rearrangements of allyl vinyl ketones.
The Meerwein–Ponndorf–Verley (MPV) reduction in organic chemistry is the reduction of ketones and aldehydes to their corresponding alcohols utilizing aluminium alkoxide catalysis in the presence of a sacrificial alcohol. The advantages of the MPV reduction lie in its high chemoselectivity and its use of a cheap environmentally friendly metal catalyst. MPV reductions have been described as "obsolete" owing to the development of sodium borohydride and related reagents.
In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, resulting in an enantioenriched sample of the less reactive enantiomer. As opposed to chiral resolution, kinetic resolution does not rely on different physical properties of diastereomeric products, but rather on the different chemical properties of the racemic starting materials. The enantiomeric excess (ee) of the unreacted starting material continually rises as more product is formed, reaching 100% just before full completion of the reaction. Kinetic resolution relies upon differences in reactivity between enantiomers or enantiomeric complexes.
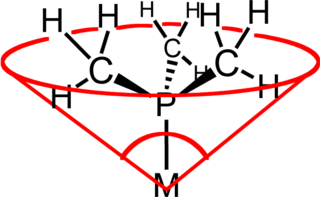
In coordination chemistry, the ligand cone angle (θ) is a measure of the steric bulk of a ligand in a transition metal coordination complex. It is defined as the solid angle formed with the metal at the vertex of a cone and the outermost edge of the van der Waals spheres of the ligand atoms at the perimeter of the base of the cone. Tertiary phosphine ligands are commonly classified using this parameter, but the method can be applied to any ligand. The term cone angle was first introduced by Chadwick A. Tolman, a research chemist at DuPont. Tolman originally developed the method for phosphine ligands in nickel complexes, determining them from measurements of accurate physical models.
The Hajos–Parrish–Eder–Sauer–Wiechert and Barbas-List reactions in organic chemistry are a family of proline-catalysed asymmetric aldol reactions.
Henri Boris Kagan is currently an emeritus professor at the Université Paris-Sud in France. He is widely recognized as a pioneer in the field of asymmetric catalysis. His discoveries have had far-reaching impacts on the pharmaceutical industry.

In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed ortho metalation is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals.
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen to a target (substrate) molecule with three-dimensional spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from other reagents or catalysts present in the reaction. This allows spatial information to transfer from one molecule to the target, forming the product as a single enantiomer. The chiral information is most commonly contained in a catalyst and, in this case, the information in a single molecule of catalyst may be transferred to many substrate molecules, amplifying the amount of chiral information present. Similar processes occur in nature, where a chiral molecule like an enzyme can catalyse the introduction of a chiral centre to give a product as a single enantiomer, such as amino acids, that a cell needs to function. By imitating this process, chemists can generate many novel synthetic molecules that interact with biological systems in specific ways, leading to new pharmaceutical agents and agrochemicals. The importance of asymmetric hydrogenation in both academia and industry contributed to two of its pioneers — William Standish Knowles and Ryōji Noyori — being collectively awarded one half of the 2001 Nobel Prize in Chemistry.

Jacobsen's catalyst is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a coordination compound of manganese and a salen-type ligand. It is used as an asymmetric catalyst in the Jacobsen epoxidation, which is renowned for its ability to enantioselectively transform prochiral alkenes into epoxides. Before its development, catalysts for the asymmetric epoxidation of alkenes required the substrate to have a directing functional group, such as an alcohol as seen in the Sharpless epoxidation. This compound has two enantiomers, which give the appropriate epoxide product from the alkene starting material.

DuPhos is a class of organophosphorus compound that are used ligands for asymmetric synthesis. The name DuPhos is derived from (1) the chemical company that sponsored the research leading to this ligand's invention, DuPont and (2) the compound is a diphosphine ligand type. Specifically it is classified as a C2-symmetric ligand, consisting of two phospholanes rings affixed to a benzene ring.
In organic chemistry, the Baylis–Hillman, Morita–Baylis–Hillman, or MBH reaction is a carbon-carbon bond-forming reaction between an activated alkene and a carbon electrophile in the presence of a nucleophilic catalyst, such as a tertiary amine or phosphine. The product is densely functionalized, joining the alkene at the α-position to a reduced form of the electrophile.
In enantioselective synthesis, a non-linear effect refers to a process in which the enantiopurity of the catalyst or chiral auxiliary does not correspond with the enantiopurity of the product produced. For example: a racemic catalyst would be expected to convert a prochiral substrate into a racemic product, but this is not always the case and a chirally enriched product can be produced instead.

Hydrogen-bond catalysis is a type of organocatalysis that relies on use of hydrogen bonding interactions to accelerate and control organic reactions. In biological systems, hydrogen bonding plays a key role in many enzymatic reactions, both in orienting the substrate molecules and lowering barriers to reaction. The field is relatively undeveloped compared to research in Lewis acid catalysis.

Trisoxazolines are a class of tridentate, chiral ligands composed of three oxazoline rings. Despite being neutral they are able to form stable complexes with high oxidation state metals, such as rare earths, due to the chelate effect. The ligands have been investigated for molecular recognition and their complexes are used in asymmetric catalysts and polymerisation.
In organic chemistry, the Keck asymmetric allylation is a chemical reaction that involves the nucleophilic addition of an allyl group to an aldehyde. The catalyst is a chiral complex that contains titanium as a Lewis acid. The chirality of the catalyst induces a stereoselective addition, so the secondary alcohol of the product has a predictable absolute stereochemistry based on the choice of catalyst. This name reaction is named for Gary Keck.
Donna Blackmond is an American chemical engineer and the John C. Martin Endowed Chair in Chemistry at Scripps Research in La Jolla, CA. Her research focuses on prebiotic chemistry, the origin of biological homochirality, and kinetics and mechanisms of asymmetric catalytic reactions. Notable works include the development of Reaction Progress Kinetic Analysis (RPKA), analysis of non-linear effects of catalyst enantiopurity, biological homochirality and amino acid behavior.
In homogeneous catalysis, C2-symmetric ligands refer to ligands that lack mirror symmetry but have C2 symmetry. Such ligands are usually bidentate and are valuable in catalysis. The C2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. C2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including C2-symmetric ligands, combine with metals or other groups to form chiral catalysts. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product.
Heterobimetallic catalysis is an approach to catalysis that employs two different metals to promote a chemical reaction. Included in this definition are cases where: 1) each metal activates a different substrate, 2) both metals interact with the same substrate, and 3) only one metal directly interacts with the substrate(s), while the second metal interacts with the first.
References
- ↑ Puchot, C.; Samuel, O.; Dunach, E.; Zhao, S. (1986). "Nonlinear effects in asymmetric synthesis. Examples in asymmetric oxidations and aldolization reactions". J. Am. Chem. Soc. 108 (9): 2353–2357. doi:10.1021/ja00269a036. PMID 22175583.
- ↑ Guillaneux, D.; Zhao, S.-H.; Samuel, O.; Rainford, D. (October 1994). "Nonlinear Effects in Asymmetric Catalysis". J. Am. Chem. Soc. 116 (21): 9430–9439. doi:10.1021/ja00100a004.
- ↑ Geiger, Y.; Achard, T.; Maisse-François, A.; Bellemin-Laponnaz, S. (May 2020). "Hyperpositive nonlinear effects in asymmetric catalysis". Nature Catalysis. 3 (5): 422–426. doi:10.1038/s41929-020-0441-1. ISSN 2520-1158 . Retrieved 2022-08-30.
- 1 2 Geiger, Yannick; Achard, Thierry; Maisse-François, Aline; Bellemin-Laponnaz, Stéphane (2020). "Hyperpositive non-linear effects: enantiodivergence and modelling". Chemical Science. 11 (46): 12453–12463. doi:10.1039/D0SC04724D. ISSN 2041-6520. PMC 8163304 . PMID 34094450 . Retrieved 2024-10-14.