Related Research Articles
In chemistry, perxenates are salts of the yellow xenon-containing anion XeO4−
6. This anion has octahedral molecular geometry, as determined by Raman spectroscopy, having O–Xe–O bond angles varying between 87° and 93°. The Xe–O bond length was determined by X-ray crystallography to be 1.875 Å.

Dichlorine monoxide is an inorganic compound with the molecular formula Cl2O. It was first synthesised in 1834 by Antoine Jérôme Balard, who along with Gay-Lussac also determined its composition. In older literature it is often referred to as chlorine monoxide, which can be a source of confusion as that name now refers to the neutral species ClO.

Chlorous acid is an inorganic compound with the formula HClO2. It is a weak acid. Chlorine has oxidation state +3 in this acid. The pure substance is unstable, disproportionating to hypochlorous acid (Cl oxidation state +1) and chloric acid (Cl oxidation state +5):

Hydrogen iodide is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent.

Iodic acid, is a white water-soluble solid with the chemical formula HIO3. Its robustness contrasts with the instability of chloric acid and bromic acid. Iodic acid features iodine in the oxidation state +5 and is one of the most stable oxo-acids of the halogens. When heated, samples dehydrate to give iodine pentoxide. On further heating, the iodine pentoxide further decomposes, giving a mix of iodine, oxygen and lower oxides of iodine.

Periodic acid is the highest oxoacid of iodine, in which the iodine exists in oxidation state +7. Like all periodates it can exist in two forms: orthoperiodic acid, with the chemical formula H5IO6 and metaperiodic acid, which has the formula HIO4.
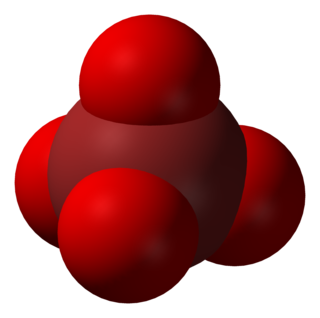
In chemistry, the perbromate ion is the anion having the chemical formula BrO−
4. It is an oxyanion of bromine, the conjugate base of perbromic acid, in which bromine has the oxidation state +7. Unlike its chlorine and iodine analogs, it is difficult to synthesize. It has tetrahedral molecular geometry.
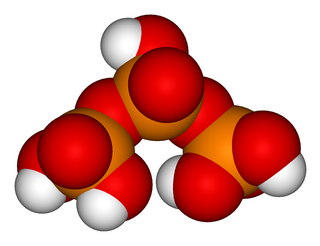
Triphosphoric acid (also tripolyphosphoric acid), with formula H5P3O10, is a condensed form of phosphoric acid. In the family of phosphoric acids, it is the next polyphosphoric acid after pyrophosphoric acid, H4P2O7, also called diphosphoric acid.

Hypobromous acid is a weak, unstable acid with chemical formula of HOBr. It is mainly produced and handled in an aqueous solution. It is generated both biologically and commercially as a disinfectant. Salts of hypobromite are rarely isolated as solids.

Phosphorus trioxide is the chemical compound with the molecular formula P4O6. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. This colorless solid is structurally related to adamantane. It is formally the anhydride of phosphorous acid, H3PO3, but cannot be obtained by the dehydration of the acid. A white solid that melts at room temperature, it is waxy, crystalline and highly toxic, with garlic odour.
Hypoiodous acid is the inorganic compound with the chemical formula HIO. It forms when an aqueous solution of iodine is treated with mercuric or silver salts. It rapidly decomposes by disproportionation:
Diimide, also called diazene or diimine, is a compound having the formula (NH)2. It exists as two geometric isomers, E (trans) and Z (cis). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is an example of an organic diazene.

Bromous acid is the inorganic compound with the formula of HBrO2. It is an unstable compound, although salts of its conjugate base – bromites – have been isolated. In acidic solution, bromites decompose to bromine.
Polonium hydride (also known as polonium dihydride, hydrogen polonide, or polane) is a chemical compound with the formula PoH2. It is a liquid at room temperature, the second hydrogen chalcogenide with this property after water. It is very unstable chemically and tends to decompose into elemental polonium and hydrogen; like all polonium compounds, it is highly radioactive. It is a volatile and very labile compound, from which many polonides can be derived.
Polysilicon hydrides are polymers containing only silicon and hydrogen. They have the formula where 0.2 ≤ n ≤ 2.5 and x is the number of monomer units. The polysilicon hydrides are generally colorless or pale-yellow/ocher powders that are easily hydrolyzed and ignite readily in air. The surfaces of silicon prepared by MOCVD using silane (SiH4) consist of a polysilicon hydride.

Polonium dioxide (also known as polonium(IV) oxide) is a chemical compound with the formula PoO2. It is one of three oxides of polonium, the other two being polonium monoxide (PoO) and polonium trioxide (PoO3). It is a pale yellow crystalline solid at room temperature. Under lowered pressure (such as a vacuum), it decomposes into elemental polonium and oxygen at 500 °C. It is the most stable oxide of polonium and is an interchalcogen.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
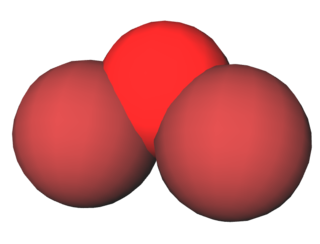
Dibromine monoxide is the chemical compound composed of bromine and oxygen with the formula Br2O. It is a dark brown solid which is stable below −40 °C and is used in bromination reactions. It is similar to dichlorine monoxide, the monoxide of its halogen neighbor one period higher on the periodic table. The molecule is bent, with C2v molecular symmetry. The Br−O bond length is 1.85Å and the Br−O−Br bond angle is 112°, similar to dichlorine monoxide.
A halous acid, also known as a halogenous acid, is an oxyacid consisting of a halogen atom in the +3 oxidation state single-bonded to a hydroxyl group and double-bonded to an oxygen atom. Examples include chlorous acid, bromous acid, and iodous acid. The conjugate base is a halite.
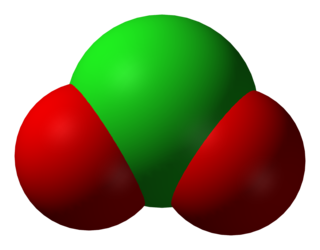
A halite, also known as a halogenite, is an oxyanion containing a halogen in a III oxidation state. It is the conjugate base of a halous acid. The known halites are chlorite, bromite, and iodite.
References
- ↑ W. Poll; G. Pawelke; D. Mootz; E. H. Appelman (1988). "The Crystal Structure of Hypofluorous Acid : Chain Formation by O−H···O Hydrogen Bonds". Angew. Chem. Int. Ed. Engl. 27 (3): 392–3. doi:10.1002/anie.198803921.
- ↑ Inorganic chemistry, Egon Wiberg, Nils Wiberg, Arnold Frederick Holleman, "Hypochlorous acid" p.442, section 4.3.1
- ↑ Holleman, A. F.; Wiberg, Egon; Wiberg, Nils (2001). Inorganic Chemistry. Academic Press. p. 451. ISBN 9780123526519 . Retrieved 24 September 2018.
- ↑ Gonick, Larry; Criddle, Craig (2005-05-03). "Chapter 9 Acid Basics". The cartoon guide to chemistry (1st ed.). HarperResource. p. 189. ISBN 9780060936778.
Similarly, we add HOCl to swimming pools to kill bacteria.
- ↑ Harris, Daniel C. (2009). Exploring Chemical Analysis (Fourth ed.). p. 538.
- ↑ Holleman, A. F.; Wiberg, Egon; Wiberg, Nils (2001). Inorganic Chemistry. Academic Press. p. 449. ISBN 9780123526519 . Retrieved 7 October 2018.
- ↑ "Oxoacids | Introduction to Chemistry". courses.lumenlearning.com. Retrieved 26 February 2019.
HOCl pKa = 7.5 < HOBr pKa = 8.6 < HOI pKa = 10.6