Related Research Articles
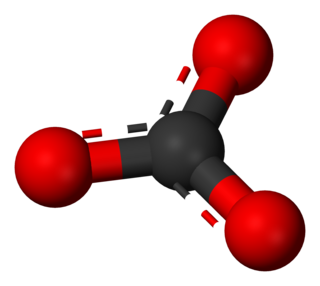
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula CO2−3. The word carbonate may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2.

A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei, and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.

Sir Frederick Augustus Abel, 1st Baronet was an English chemist who was recognised as the leading British authority on explosives. He is best known for the invention of cordite as a replacement for gunpowder in firearms.
Nitric acid is the inorganic compound with the formula HNO3. It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%.
Geochemistry is the science that uses the tools and principles of chemistry to explain the mechanisms behind major geological systems such as the Earth's crust and its oceans. The realm of geochemistry extends beyond the Earth, encompassing the entire Solar System, and has made important contributions to the understanding of a number of processes including mantle convection, the formation of planets and the origins of granite and basalt. It is an integrated field of chemistry and geology.

Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula B(OH)3. It may also be called hydrogen borate, boron hydroxide or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salts, and can react with alcohols to form borate esters.

Weathering is the deterioration of rocks, soils and minerals as well as wood and artificial materials through contact with water, atmospheric gases, and biological organisms. Weathering occurs in situ, and so is distinct from erosion, which involves the transport of rocks and minerals by agents such as water, ice, snow, wind, waves and gravity.

Quercitron is a yellow natural dye obtained from the bark of the Eastern Black Oak, a forest tree indigenous in North America. It was formerly called Dutch pink, English pink, or Italian pink.

Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides.

Potassium alum, potash alum, or potassium aluminium sulfate is a chemical compound: the double sulfate of potassium and aluminium, with chemical formula KAl(SO4)2. It is commonly encountered as the dodecahydrate, KAl(SO4)2·12H2O. It crystallizes in an octahedral structure in neutral solution and cubic structure in an alkali solution with space group P a −3 and lattice parameter of 12.18 Å. The compound is the most important member of the generic class of compounds called alums, and is often called simply alum.

Charles Adolphe Wurtz was an Alsatian French chemist. He is best remembered for his decades-long advocacy for the atomic theory and for ideas about the structures of chemical compounds, against the skeptical opinions of chemists such as Marcellin Berthelot and Henri Étienne Sainte-Claire Deville. He is well known by organic chemists for the Wurtz reaction, to form carbon-carbon bonds by reacting alkyl halides with sodium, and for his discoveries of ethylamine, ethylene glycol, and the aldol reaction. Wurtz was also an influential writer and educator.

Terrestrial rocks are formed by three main mechanisms:

Mucic acid, C6H10O8 or HOOC-(CHOH)4-COOH (also known as galactaric or meso-galactaric acid) is an aldaric acid obtained by nitric acid oxidation of galactose or galactose-containing compounds such as lactose, dulcite, quercite, and most varieties of gum.

Victor Moritz Goldschmidt was a Norwegian mineralogist considered to be the founder of modern geochemistry and crystal chemistry, developer of the Goldschmidt Classification of elements.

Aluminium sulfate is a salt with the formula Al2(SO4)3. It is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge) in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing.

Eilhard Mitscherlich was a German chemist, who is perhaps best remembered today for his discovery of the phenomenon of crystallographic isomorphism in 1819.

Levulinic acid, or 4-oxopentanoic acid, is an organic compound with the formula CH3C(O)CH2CH2CO2H. It is classified as a keto acid. This white crystalline solid is soluble in water and polar organic solvents. It is derived from degradation of cellulose and is a potential precursor to biofuels, such as ethyl levulinate.

Orcinol is an organic compound with the formula CH3C6H3(OH)2. It occurs in many species of lichens including Roccella tinctoria and Lecanora. Orcinol has been detected in the "toxic glue" of the ant species Camponotus saundersi. It is a colorless solid. It is related to resorcinol, 1,3-C6H4(OH)2.
Animal nutrition focuses on the dietary nutrients needs of animals, primarily those in agriculture and food production, but also in zoos, aquariums, and wildlife management.

Foliose lichen is one of the morphological classes of lichens, which are complex organisms that arise from the symbiotic relationship between fungi and a photosynthetic partner, typically algae. This partnership allows lichen to live in diverse climates that can range from cold, dry mountains to wet, warm valleys. Lichens develop quite slowly with recorded growth rates of 0.01–27mm/year depending on the species. Their lifespan averages between 30 and 60 years.
References
- ↑ Thomson, Thomas (1838). Chemistry of Organic Bodies: Vegetables. Maclachlan & Stewart. p. 748.
- ↑ Chisholm 1911.
- ↑ Goldschmidt, G. (1879). Watts, Henry (ed.). Journal of the Chemical Society. The Chemical Society of London. p. 167.
- This article incorporates text from a publication now in the public domain : Chisholm, Hugh, ed. (1911). "Idrialin". Encyclopædia Britannica . Vol. 14 (11th ed.). Cambridge University Press. p. 289.