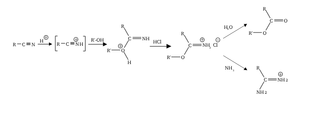
The Pinner reaction refers to the acid catalysed reaction of a nitrile with an alcohol to form an imino ester salt ; this is sometimes referred to as a Pinner salt. The reaction is named after Adolf Pinner, who first described it in 1877. Pinner salts are themselves reactive and undergo additional nucleophilic additions to give various useful products:
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions differ from electrophilic additions in that the former reactions involve the group to which atoms are added accepting electron pairs, whereas the latter reactions involve the group donating electron pairs.

An imine is a functional group or chemical compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen (H) or an organic group (R). If this group is not a hydrogen atom, then the compound can sometimes be referred to as a Schiff base. The carbon atom has two additional single bonds. The term "imine" was coined in 1883 by the German chemist Albert Ladenburg.

A Schiff base (named after Hugo Schiff) is a compound with the general structure R1R2C=NR' (R' ≠ H). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimines depending on their structure. The term is often synonymous with azomethine which refers specifically to secondary aldimines (i.e. R-CH=NR' where R' ≠ H).
A nitrile is any organic compound that has a −C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after chemist Carl Mannich.

Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in specialized syntheses.
1,3,5-Triazine, also called s-triazine, is an organic chemical compound with the formula (HCN)3. It is a six-membered heterocyclic aromatic ring, one of several isomeric triazines. S-triazine—the "symmetric" isomer—and its derivatives are useful in a variety of applications.

The Doebner–Miller reaction is the organic reaction of an aniline with α,β-unsaturated carbonyl compounds to form quinolines.
Nitrilimines or nitrile amides are a class of organic compounds sharing a common functional group with the general structure R-CN-NR corresponding to the conjugate base of an amine bonded to the N-terminus of a nitrile. The dominant structure for the parent compound nitrilimine is that of the propargyl-like 1 in scheme 1 with a C-N triple bond and with a formal positive charge on nitrogen and two lone pairs and a formal negative charge on the terminal nitrogen. Other structures such as hypervalent 2, allene-like 3, allylic 4 and carbene 5 are of lesser relevance.
The Cook–Heilbron thiazole synthesis highlights the formation of 5-aminothiazoles through the chemical reaction of α-aminonitriles or aminocynoacetates with dithioacids, carbon disulphide, carbon oxysulfide, or isothiocynates at room temperature and under mild or aqueous conditions. Variation of substituents at the 2nd and 4th position of the thiazole is introduced by selecting different combinations of starting reagents.

Sydnones are mesoionic heterocyclic chemical compounds possessing a 1,2,3-oxadiazole core with a keto group in the 5 position. Like other mesoionic compounds they are di-polar, possessing both positive and negative charges which are delocalized across the ring. Recent computational studies have indicated that sydnones and other similar mesoionic compounds are nonaromatic, "though well-stabilized in two separate regions by electron and charge delocalization." Sydnones are a heterocyclic compound named after the city of Sydney, Australia.
The Stieglitz rearrangement is a rearrangement reaction in organic chemistry which is named after the American chemist Julius Stieglitz (1867–1937) and was first investigated by him and Paul Nicholas Leech in 1913. It describes the 1,2-rearrangement of trityl amine derivatives to triaryl imines. It is comparable to a Beckmann rearrangement which also involves a substitution at a nitrogen atom through a carbon to nitrogen shift. As an example, triaryl hydroxylamines can undergo a Stieglitz rearrangement by dehydration and the shift of a phenyl group after activation with phosphorus pentachloride to yield the respective triaryl imine, a Schiff base.

Sir Jocelyn Field Thorpe FRS was a British chemist who made major contributions to organic chemistry, including the Thorpe-Ingold effect and three named reactions.
The Hoesch reaction or Houben–Hoesch reaction is an organic reaction in which a nitrile reacts with an arene compound to form an aryl ketone. The reaction is a type of Friedel-Crafts acylation with hydrogen chloride and a Lewis acid catalyst.
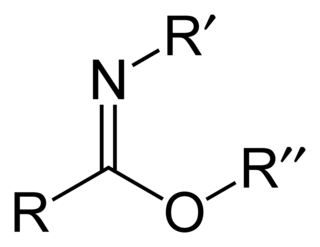
Carboximidates are organic compounds, which can be thought of as esters formed between a carboximidic acid and an alcohol, with the general formula R-C(=NR')OR".
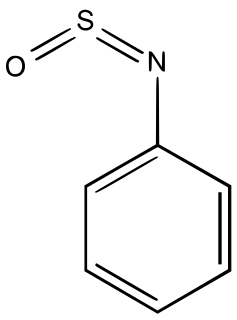
Sulfinylamines are organosulfur compounds with the formula RNSO where R = an organic substituent. These compounds are, formally speaking, derivatives of HN=S=O, i.e. analogues of sulfur dioxide and of sulfur diimide. A common example is N-sulfinylaniline. Sulfinyl amines are dienophile. They undergo [2+2] cycloaddition to ketenes.

Hydroxylamine-O-sulfonic acid (HOSA) is the inorganic compound with molecular formula H3NO4S that is formed by the sulfonation of hydroxylamine with oleum. It is a white, water-soluble and hygroscopic, solid, commonly represented by the condensed structural formula H2NOSO3H, though it actually exists as a zwitterion and thus is more accurately represented as +H3NOSO3−. It is used as a reagent for the introduction of amine groups (–NH2), for the conversion of aldehydes into nitriles and alicyclic ketones into lactams (cyclic amides), and for the synthesis of variety of nitrogen-containing heterocycles.










