In vitro studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in biology and its subdisciplines are traditionally done in labware such as test tubes, flasks, Petri dishes, and microtiter plates. Studies conducted using components of an organism that have been isolated from their usual biological surroundings permit a more detailed or more convenient analysis than can be done with whole organisms; however, results obtained from in vitro experiments may not fully or accurately predict the effects on a whole organism. In contrast to in vitro experiments, in vivo studies are those conducted in living organisms, including humans, known as clinical trials, and whole plants.

In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can differentiate into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type of cell in a cell lineage. They are found in both embryonic and adult organisms, but they have slightly different properties in each. They are usually distinguished from progenitor cells, which cannot divide indefinitely, and precursor or blast cells, which are usually committed to differentiating into one cell type.

A genetic chimerism or chimera is a single organism composed of cells with more than one distinct genotype. In animals and human chimeras, this means an individual derived from two or more zygotes, which can include possessing blood cells of different blood types, and subtle variations in form (phenotype). Animal chimeras are produced by the merger of two embryos. In plant chimeras, however, the distinct types of tissue may originate from the same zygote, and the difference is often due to mutation during ordinary cell division. Normally, genetic chimerism is not visible on casual inspection; however, it has been detected in the course of proving parentage. In contrast, an individual where each cell contains genetic material from two organisms of different breeds, varieties, species or genera is called a hybrid.
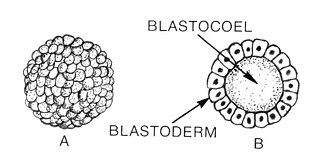
Blastulation is the stage in early animal embryonic development that produces the blastula. In mammalian development the blastula develops into the blastocyst with a differentiated inner cell mass and an outer trophectoderm. The blastula is a hollow sphere of cells known as blastomeres surrounding an inner fluid-filled cavity called the blastocoel. Embryonic development begins with a sperm fertilizing an egg cell to become a zygote, which undergoes many cleavages to develop into a ball of cells called a morula. Only when the blastocoel is formed does the early embryo become a blastula. The blastula precedes the formation of the gastrula in which the germ layers of the embryo form.
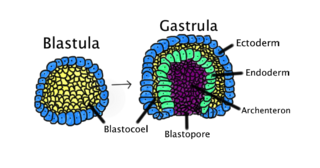
Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst is reorganized into a two-layered or three-layered embryo known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.

Serum is the fluid and solute component of blood which does not play a role in clotting. It may be defined as blood plasma without the clotting factors, or as blood with all cells and clotting factors removed. Serum includes all proteins not used in blood clotting; all electrolytes, antibodies, antigens, hormones; and any exogenous substances. Serum does not contain white blood cells (leukocytes), red blood cells (erythrocytes), platelets, or clotting factors.

The blastocyst is a structure formed in the early embryonic development of mammals. It possesses an inner cell mass (ICM) also known as the embryoblast which subsequently forms the embryo, and an outer layer of trophoblast cells called the trophectoderm. This layer surrounds the inner cell mass and a fluid-filled cavity known as the blastocoel. In the late blastocyst the trophectoderm is known as the trophoblast. The trophoblast gives rise to the chorion and amnion, the two fetal membranes that surround the embryo. The placenta derives from the embryonic chorion and the underlying uterine tissue of the mother.
Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the inner cell mass (embryoblast) using immunosurgery results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage have the same moral considerations as embryos in the post-implantation stage of development.

Embryonic diapause (delayed implantation in mammals) is a reproductive strategy used by a number of animal species across different biological classes. In more than 130 types of mammals where this takes place, the process occurs at the blastocyst stage of embryonic development, and is characterized by a dramatic reduction or complete cessation of mitotic activity, arresting most often in the G0 or G1 phase of division.

Embryoid bodies (EBs) are three-dimensional aggregates of pluripotent stem cells.
James Alexander Thomson is an American developmental biologist best known for deriving the first human embryonic stem cell line in 1998 and for deriving human induced pluripotent stem cells (iPS) in 2007.

A stem cell line is a group of stem cells that is cultured in vitro and can be propagated indefinitely. Stem cell lines are derived from either animal or human tissues and come from one of three sources: embryonic stem cells, adult stem cells, or induced stem cells. They are commonly used in research and regenerative medicine.

The inner cell mass (ICM) or embryoblast is a structure in the early development of an embryo. It is the mass of cells inside the blastocyst that will eventually give rise to the definitive structures of the fetus. The inner cell mass forms in the earliest stages of embryonic development, before implantation into the endometrium of the uterus. The ICM is entirely surrounded by the single layer of trophoblast cells of the trophectoderm.
In molecular cloning and biology, a gene knock-in refers to a genetic engineering method that involves the one-for-one substitution of DNA sequence information in a genetic locus or the insertion of sequence information not found within the locus. Typically, this is done in mice since the technology for this process is more refined and there is a high degree of shared sequence complexity between mice and humans. The difference between knock-in technology and traditional transgenic techniques is that a knock-in involves a gene inserted into a specific locus, and is thus a "targeted" insertion. It is the opposite of gene knockout.

Janet Rossant, is a developmental biologist well known for her contributions to the understanding of the role of genes in embryo development. She is a world renowned leader in developmental biology. Her current research interests focus on stem cells, molecular genetics, and developmental biology. Specifically, she uses cellular and genetic manipulation techniques to study how genes control both normal and abnormal development of early mouse embryos. Rossant has discovered information on embryo development, how multiple types of stem cells are established, and the mechanisms by which genes control development. In 1998, her work helped lead to the discovery of the trophoblast stem cell, which has assisted in showing how congenital anomalies in the heart, blood vessels, and placenta can occur.

Cell potency is a cell's ability to differentiate into other cell types. The more cell types a cell can differentiate into, the greater its potency. Potency is also described as the gene activation potential within a cell, which like a continuum, begins with totipotency to designate a cell with the most differentiation potential, pluripotency, multipotency, oligopotency, and finally unipotency.
A knockout mouse, or knock-out mouse, is a genetically modified mouse in which researchers have inactivated, or "knocked out", an existing gene by replacing it or disrupting it with an artificial piece of DNA. They are important animal models for studying the role of genes which have been sequenced but whose functions have not been determined. By causing a specific gene to be inactive in the mouse, and observing any differences from normal behaviour or physiology, researchers can infer its probable function.
Induced stem cells (iSC) are stem cells derived from somatic, reproductive, pluripotent or other cell types by deliberate epigenetic reprogramming. They are classified as either totipotent (iTC), pluripotent (iPSC) or progenitor or unipotent – (iUSC) according to their developmental potential and degree of dedifferentiation. Progenitors are obtained by so-called direct reprogramming or directed differentiation and are also called induced somatic stem cells.
After the blastocyst stage, once an embryo implanted in endometrium, the inner cell mass (ICM) of a fertilized embryo segregates into two layers: hypoblast and epiblast. The epiblast cells are the functional progenitors of soma and germ cells which later differentiate into three layers: definitive endoderm, mesoderm and ectoderm. Stem cells derived from epiblast are pluripotent. These cells are called epiblast-derived stem cells (EpiSCs) and have several different cellular and molecular characteristics with Embryonic Stem Cells (ESCs). Pluripotency in EpiSCs is essentially different from that of embryonic stem cells. The pluripotency of EpiSCs is primed pluripotency: primed to differentiate into specific cell lineages. Naïve pluripotent stem cells and primed pluripotent stem cells not only sustain the ability to self-renew but also maintain the capacity to differentiate. Since the cell status is primed to differentiate in EpiSCs, however, one copy of the X chromosome in XX cells in EpiSCs is silenced (XaXi). EpiSCs is unable to colonize and is not available to be used to produce chimeras. Conversely, XX cells in ESCs are both active and can produce chimera when inserted into a blastocyst. Both ESC and EpiSC induce teratoma when injected in the test animals which proves pluripotency. EpiSC display several distinctive characteristics distinct from ESCs. The cellular status of human ESCs (hESCs) is similar to primed state mouse stem cells rather than naïve state.
Sir Richard Lavenham Gardner, FRSB, FRS is a British embryologist and geneticist. He is currently an Emeritus Professor at the University of York, and was previously a Royal Society Research Professor.