
Ultraviolet (UV) light is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs, Cherenkov radiation, and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights.

Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet, visible (400–750 nm), or infrared radiation (750–2500 nm).

Sun tanning or tanning is the process whereby skin color is darkened or tanned. It is most often a result of exposure to ultraviolet (UV) radiation from sunlight or from artificial sources, such as a tanning lamp found in indoor tanning beds. People who deliberately tan their skin by exposure to the sun engage in a passive recreational activity of sun bathing. Some people use chemical products that can produce a tanning effect without exposure to ultraviolet radiation, known as sunless tanning.

Sunless tanning, also known as UV filled tanning, self tanning, spray tanning, or fake tanning, refers to the effect of a suntan without exposure to the Sun. Sunless tanning involves the use of oral agents (carotenids), or creams, lotions or sprays applied to the skin. Skin-applied products may be skin-reactive agents or temporary bronzers (colorants).

Avobenzone is an organic molecule and an oil-soluble ingredient used in sunscreen products to absorb the full spectrum of UVA rays.

Octyl methoxycinnamate or ethylhexyl methoxycinnamate (INCI) or octinoxate (USAN), trade names Eusolex 2292 and Uvinul MC80, is an organic compound that is an ingredient in some sunscreens and lip balms. It is an ester formed from methoxycinnamic acid and 2-ethylhexanol. It is a liquid that is insoluble in water.

Psoralen is the parent compound in a family of naturally occurring organic compounds known as the linear furanocoumarins. It is structurally related to coumarin by the addition of a fused furan ring, and may be considered as a derivative of umbelliferone. Psoralen occurs naturally in the seeds of Psoralea corylifolia, as well as in the common fig, celery, parsley, West Indian satinwood, and in all citrus fruits. It is widely used in PUVA treatment for psoriasis, eczema, vitiligo, and cutaneous T-cell lymphoma; these applications are typically through the use of medications such as Methoxsalen. Many furanocoumarins are extremely toxic to fish, and some are deposited in streams in Indonesia to catch fish.

Photodegradation is the alteration of materials by light. Commonly, the term is used loosely to refer to the combined action of sunlight and air, which cause oxidation and hydrolysis. Often photodegradation is intentionally avoided, since it destroys paintings and other artifacts. It is, however, partly responsible for remineralization of biomass and is used intentionally in some disinfection technologies. Photodegradation does not apply to how materials may be aged or degraded via infrared light or heat, but does include degradation in all of the ultraviolet light wavebands.

UV filters are compounds, mixtures, or materials that block or absorb ultraviolet (UV) light. One of the major applications of UV filters is their use as sunscreens to protect skin from sunburn and other sun/UV related damage. After the invention of digital cameras changed the field of photography, UV filters have been used to coat glass discs fitted to camera lenses to protect hardware that is sensitive to UV light.
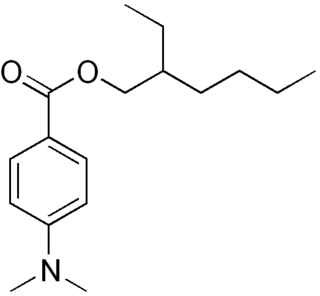
Padimate O is an organic compound related to the water-soluble compound PABA that is used as an ingredient in some sunscreens. This yellowish water-insoluble oily liquid is an ester formed by the condensation of 2-ethylhexanol with dimethylaminobenzoic acid. Other names for padimate O include 2-ethylhexyl 4-dimethylaminobenzoate, Escalol 507, octyldimethyl PABA, and OD-PABA.
Photoprotection is the biochemical process that helps organisms cope with molecular damage caused by sunlight. Plants and other oxygenic phototrophs have developed a suite of photoprotective mechanisms to prevent photoinhibition and oxidative stress caused by excess or fluctuating light conditions. Humans and other animals have also developed photoprotective mechanisms to avoid UV photodamage to the skin, prevent DNA damage, and minimize the downstream effects of oxidative stress.
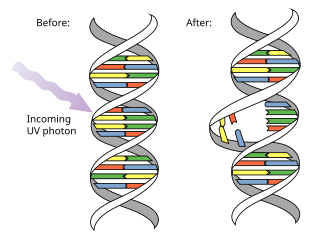
Pyrimidine dimers represent molecular lesions originating from thymine or cytosine bases within DNA, resulting from photochemical reactions. These lesions, commonly linked to direct DNA damage, are induced by ultraviolet light (UV), particularly UVC, result in the formation of covalent bonds between adjacent nitrogenous bases along the nucleotide chain near their carbon–carbon double bonds, the photo-coupled dimers are fluorescent. Such dimerization, which can also occur in double-stranded RNA (dsRNA) involving uracil or cytosine, leads to the creation of cyclobutane pyrimidine dimers (CPDs) and 6–4 photoproducts. These pre-mutagenic lesions modify the DNA helix structure, resulting in abnormal non-canonical base pairing and, consequently, adjacent thymines or cytosines in DNA will form a cyclobutane ring when joined together and cause a distortion in the DNA. This distortion prevents DNA replication and transcription mechanisms beyond the dimerization site.
A photocarcinogen is a substance which causes cancer when an organism is exposed to it, then illuminated. Many chemicals that are not carcinogenic can be photocarcinogenic when combined with exposure to light, especially UV. This can easily be understood from a photochemical perspective: The reactivity of a chemical substance itself might be low, but after illumination it transitions to an excited state, which is chemically much more reactive and therefore potentially harmful to biological tissue and DNA. Light can also split photocarcinogens, releasing free radicals, whose unpaired electrons cause them to be extremely reactive.
Tanning activators are chemicals that increase the effect of UV-radiation on the human skin.

In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.

Photoaging or photoageing is a term used for the characteristic changes to skin induced by chronic UVA and UVB exposure. Tretinoin is the best studied retinoid in the treatment of photoaging.
Mycosporine-like amino acids (MAAs) are small secondary metabolites produced by organisms that live in environments with high volumes of sunlight, usually marine environments. The exact number of compounds within this class of natural products is yet to be determined, since they have only relatively recently been discovered and novel molecular species are constantly being discovered; however, to date their number is around 30. They are commonly described as “microbial sunscreens” although their function is believed not to be limited to sun protection. MAAs represent high potential in cosmetics, and biotechnological applications. Indeed, their UV-absorbing properties would allow to create products derived from natural photoprotectors, potentially harmless to the environment and efficient against UV damage.

Angelicin is the parent compound in a family of naturally occurring organic compounds known as the angular furanocoumarins. Structurally, it can be considered as benzapyra-2-one fused with a furan moiety in the 7,8-position. Angelicin is commonly found in certain Apiaceae and Fabaceae plant species such as Bituminaria bituminosa. It has a skin permeability coefficient (LogKp) of -2.46. The maximum absorption is observed at 300 nm. The 1HNMR spectrum is available; the infrared and mass spectra of angelicin can be found in this database. The sublimation of angelicin occurs at 120 °C and the pressure of 0.13 Pa. Angelicin is a coumarin.
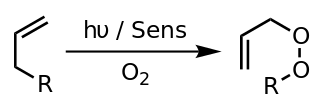
A photooxygenation is a light-induced oxidation reaction in which molecular oxygen is incorporated into the product(s). Initial research interest in photooxygenation reactions arose from Oscar Raab's observations in 1900 that the combination of light, oxygen and photosensitizers is highly toxic to cells. Early studies of photooxygenation focused on oxidative damage to DNA and amino acids, but recent research has led to the application of photooxygenation in organic synthesis and photodynamic therapy.

Dimitra Markovitsi is a Greek-French photochemist. She is currently an Emeritus Research Director at the French National Center for Scientific Research (CNRS). She pioneered studies on the electronically excited states of liquid crystals and made significant advances to the understanding of processes triggered in DNA upon absorption of UV radiation. The two facets of her work have been the subject of a recent Marie Skodowska Curie European training network entitled "Light DyNAmics - DNA as a training platform for photodynamic processes in soft materials."


















