This article relies largely or entirely on a single source .(April 2024) |

An ion-to-photon detector (IPD) is a component used for detecting ions in mass spectrometry.
This article relies largely or entirely on a single source .(April 2024) |

An ion-to-photon detector (IPD) is a component used for detecting ions in mass spectrometry.
In an ion-to-photon detector, a photomultiplier tube is coated with a layer of scintillator compound, such as Rhodamine B or CsI. When the ions pass through the mass analyzer of the spectrometer, they strike the scintillator compound and cause the release of photons. These photons are then detected by the photomultiplier tube. A conversion dynode, such as a microchannel plate can also be used between the ion beam and the scintillator to increase the signal. An MCP, when struck by an ion, will release electrons which then strike the scintillator. [1]
The primary application for ion-to-photon detectors is acting as the detector in MALDI mass spectrometry. [1] They could, in theory, be used for other types of mass spectrometry as well.
The conversion efficiency of ions to photons by an IPD is as good as, or better than, the conversion efficiency of ions to electrons by a multichannel plate detector. Ion-to-photon detectors may also detect ions with a mass of up to around 20,000 Da, better than microchannel plates. However, the resolution of the mass spectrum from an IPD equipped spectrometer is slightly lower. The noise in the spectrum, which may come from unfocused, slow speed ions, is also slightly higher. [1]

X-ray fluorescence (XRF) is the emission of characteristic "secondary" X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science, archaeology and art objects such as paintings.

Photomultiplier tubes (photomultipliers or PMTs for short) are extremely sensitive detectors of light in the ultraviolet, visible, and near-infrared ranges of the electromagnetic spectrum. They are members of the class of vacuum tubes, more specifically vacuum phototubes. These detectors multiply the current produced by incident light by as much as 100 million times or 108 (i.e., 160 dB), in multiple dynode stages, enabling (for example) individual photons to be detected when the incident flux of light is low.

A scintillation counter is an instrument for detecting and measuring ionizing radiation by using the excitation effect of incident radiation on a scintillating material, and detecting the resultant light pulses.

Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.

A scintillator is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate. Sometimes, the excited state is metastable, so the relaxation back down from the excited state to lower states is delayed. The process then corresponds to one of two phenomena: delayed fluorescence or phosphorescence. The correspondence depends on the type of transition and hence the wavelength of the emitted optical photon.

A photocathode is a surface engineered to convert light (photons) into electrons using the photoelectric effect. Photocathodes are important in accelerator physics where they are utilised in a photoinjector to generate high brightness electron beams. Electron beams generated with photocathodes are commonly used for free electron lasers and for ultrafast electron diffraction. Photocathodes are also commonly used as the negatively charged electrode in a light detection device such as a photomultiplier, phototube and image intensifier.
Liquid scintillation counting is the measurement of radioactive activity of a sample material which uses the technique of mixing the active material with a liquid scintillator, and counting the resultant photon emissions. The purpose is to allow more efficient counting due to the intimate contact of the activity with the scintillator. It is generally used for alpha particle or beta particle detection.

Gas chromatography–mass spectrometry (GC–MS) is an analytical method that combines the features of gas-chromatography and mass spectrometry to identify different substances within a test sample. Applications of GC–MS include drug detection, fire investigation, environmental analysis, explosives investigation, food and flavor analysis, and identification of unknown samples, including that of material samples obtained from planet Mars during probe missions as early as the 1970s. GC–MS can also be used in airport security to detect substances in luggage or on human beings. Additionally, it can identify trace elements in materials that were previously thought to have disintegrated beyond identification. Like liquid chromatography–mass spectrometry, it allows analysis and detection even of tiny amounts of a substance.

X-ray spectroscopy is a general term for several spectroscopic techniques for characterization of materials by using x-ray radiation.

Photodetectors, also called photosensors, are sensors of light or other electromagnetic radiation. There are a wide variety of photodetectors which may be classified by mechanism of detection, such as photoelectric or photochemical effects, or by various performance metrics, such as spectral response. Semiconductor-based photodetectors typically use a p–n junction that converts photons into charge. The absorbed photons make electron–hole pairs in the depletion region. Photodiodes and photo transistors are a few examples of photo detectors. Solar cells convert some of the light energy absorbed into electrical energy.
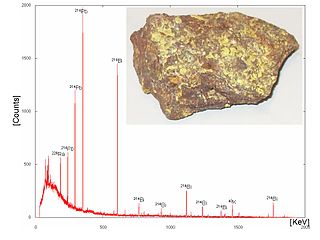
Gamma-ray spectroscopy is the qualitative study of the energy spectra of gamma-ray sources, such as in the nuclear industry, geochemical investigation, and astrophysics. Gamma-ray spectrometry, on the other hand, is the method used to acquire a quantitative spectrum measurement.
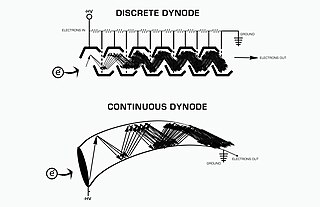
An electron multiplier is a vacuum-tube structure that multiplies incident charges. In a process called secondary emission, a single electron can, when bombarded on secondary-emissive material, induce emission of roughly 1 to 3 electrons. If an electric potential is applied between this metal plate and yet another, the emitted electrons will accelerate to the next metal plate and induce secondary emission of still more electrons. This can be repeated a number of times, resulting in a large shower of electrons all collected by a metal anode, all having been triggered by just one.

Neutron detection is the effective detection of neutrons entering a well-positioned detector. There are two key aspects to effective neutron detection: hardware and software. Detection hardware refers to the kind of neutron detector used and to the electronics used in the detection setup. Further, the hardware setup also defines key experimental parameters, such as source-detector distance, solid angle and detector shielding. Detection software consists of analysis tools that perform tasks such as graphical analysis to measure the number and energies of neutrons striking the detector.
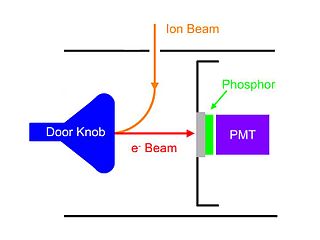
A Daly detector is a gas-phase ion detector that consists of a metal "doorknob", a scintillator and a photomultiplier. It was named after its inventor Norman Richard Daly. Daly detectors are typically used in mass spectrometers.

Time-of-flight mass spectrometry (TOFMS) is a method of mass spectrometry in which an ion's mass-to-charge ratio is determined by a time of flight measurement. Ions are accelerated by an electric field of known strength. This acceleration results in an ion having the same kinetic energy as any other ion that has the same charge. The velocity of the ion depends on the mass-to-charge ratio. The time that it subsequently takes for the ion to reach a detector at a known distance is measured. This time will depend on the velocity of the ion, and therefore is a measure of its mass-to-charge ratio. From this ratio and known experimental parameters, one can identify the ion.
Electron capture ionization is the ionization of a gas phase atom or molecule by attachment of an electron to create an ion of the form . The reaction is
PERDaix is a novel, small and light weight magnetic spectrometer to measure the charge and mass dependent solar modulation periodically for deeper understanding of cosmic rays. For a better understanding of sources and acceleration of cosmic particles direct measurements of cosmic rays are necessary. Also for a better understanding of the solar modulation which is expected to follow the 22-year solar cycle, time dependent measurements are needed. PERDaix is a newly designed detector which is constructed by the Department of Physics 1b, RWTH Aachen University. Being proposed to the German Space Agency in November 2009 for a participation in the BEXUS Program after a first canceled flight attempt in October 2010 the actual flight took place as a post-BEXUS-campaign flight opportunity in November 2010.
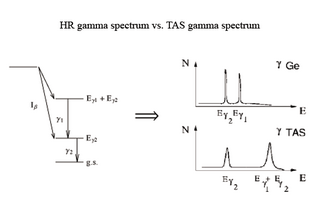
Total absorption spectroscopy is a measurement technique that allows the measurement of the gamma radiation emitted in the different nuclear gamma transitions that may take place in the daughter nucleus after its unstable parent has decayed by means of the beta decay process. This technique can be used for beta decay studies related to beta feeding measurements within the full decay energy window for nuclei far from stability.

A microchannel plate (MCP) is used to detect single particles and photons. It is closely related to an electron multiplier, as both intensify single particles or photons by the multiplication of electrons via secondary emission. Because a microchannel plate detector has many separate channels, it can provide spatial resolution.

X-ray detectors are devices used to measure the flux, spatial distribution, spectrum, and/or other properties of X-rays.