Related Research Articles

In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.

The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splinters, distinguishing them from the organism's own healthy tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use molecules and cells to perform their functions.

Immunology is a branch of biology and medicine that covers the study of immune systems in all organisms.

In immunology, autoimmunity is the system of immune responses of an organism against its own healthy cells, tissues and other normal body constituents. Any disease resulting from this type of immune response is termed an "autoimmune disease". Prominent examples include celiac disease, diabetes mellitus type 1, Henoch–Schönlein purpura, systemic lupus erythematosus, Sjögren syndrome, eosinophilic granulomatosis with polyangiitis, Hashimoto's thyroiditis, Graves' disease, idiopathic thrombocytopenic purpura, Addison's disease, rheumatoid arthritis, ankylosing spondylitis, polymyositis, dermatomyositis, and multiple sclerosis. Autoimmune diseases are very often treated with steroids.
An immune response is a physiological reaction which occurs within an organism in the context of inflammation for the purpose of defending against exogenous factors. These include a wide variety of different toxins, viruses, intra- and extracellular bacteria, protozoa, helminths, and fungi which could cause serious problems to the health of the host organism if not cleared from the body.

Natural killer cells, also known as NK cells, are a type of cytotoxic lymphocyte critical to the innate immune system. They are a kind of large granular lymphocytes (LGL), and belong to the rapidly expanding family of known innate lymphoid cells (ILC) and represent 5–20% of all circulating lymphocytes in humans. The role of NK cells is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cells, stressed cells, tumor cells, and other intracellular pathogens based on signals from several activating and inhibitory receptors. Most immune cells detect the antigen presented on major histocompatibility complex I (MHC-I) on infected cell surfaces, but NK cells can recognize and kill stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named "natural killers" because of the notion that they do not require activation to kill cells that are missing "self" markers of MHC class I. This role is especially important because harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells, such as T lymphocyte cells.
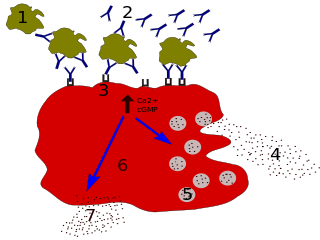
Immunoglobulin E (IgE) is a type of antibody that has been found only in mammals. IgE is synthesised by plasma cells. Monomers of IgE consist of two heavy chains and two light chains, with the ε chain containing four Ig-like constant domains (Cε1–Cε4). IgE is thought to be an important part of the immune response against infection by certain parasitic worms, including Schistosoma mansoni, Trichinella spiralis, and Fasciola hepatica. IgE is also utilized during immune defense against certain protozoan parasites such as Plasmodium falciparum. IgE may have evolved as a defense to protect against venoms.

Polly Celine Eveline Matzinger is a French-born immunologist who proposed the danger model theory of how the immune system works.
Memory T cells are a subset of T lymphocytes that might have some of the same functions as memory B cells. Their lineage is unclear.

Ellen Heber-Katz is an American immunologist and regeneration biologist who works as a professor at Lankenau Institute for Medical Research (LIMR). She discovered that the Murphy Roths Large (MRL) mouse strain can regenerate wounds without scarring and fully restore damaged tissue. Her research focuses on immunology, regenerative medicine, and cancer. In July 2015, she expanded her research to include studies funded by the National Cancer Institute (NCI) that investigate novel aspects of breast cancer causation.

Toll-like receptor 6 is a protein that in humans is encoded by the TLR6 gene. TLR6 is a transmembrane protein, member of toll-like receptor family, which belongs to the pattern recognition receptor (PRR) family. TLR6 acts in a heterodimer form with toll-like receptor 2 (TLR2). Its ligands include multiple diacyl lipopeptides derived from gram-positive bacteria and mycoplasma and several fungal cell wall saccharides. After dimerizing with TLR2, the NF-κB intracellular signalling pathway is activated, leading to a pro-inflammatory cytokine production and activation of innate immune response. TLR6 has also been designated as CD286.
Gamma delta T cells are T cells that have a γδ T-cell receptor (TCR) on their surface. Most T cells are αβ T cells with TCR composed of two glycoprotein chains called α (alpha) and β (beta) TCR chains. In contrast, γδ T cells have a TCR that is made up of one γ (gamma) chain and one δ (delta) chain. This group of T cells is usually less common than αβ T cells. Their highest abundance is in the gut mucosa, within a population of lymphocytes known as intraepithelial lymphocytes (IELs).

Hepatitis A virus cellular receptor 2 (HAVCR2), also known as T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), is a protein that in humans is encoded by the HAVCR2 (TIM-3)gene. HAVCR2 was first described in 2002 as a cell surface molecule expressed on IFNγ producing CD4+ Th1 and CD8+ Tc1 cells. Later, the expression was detected in Th17 cells, regulatory T-cells, and innate immune cells. HAVCR2 receptor is a regulator of the immune response.
The following outline is provided as an overview of and topical guide to immunology:
Immunology is the study of the immune system during health and disease. Below is a list of immunology-related articles.

Akiko Iwasaki is a Sterling Professor of Immunobiology and Molecular, Cellular and Developmental Biology at Yale University. She is also a principal investigator at the Howard Hughes Medical Institute. Her research interests include innate immunity, autophagy, inflammasomes, sexually transmitted infections, herpes simplex virus, human papillomavirus, respiratory virus infections, influenza infection, T cell immunity, commensal bacteria, COVID-19, and long COVID.

The intestinal mucosal barrier, also referred to as intestinal barrier, refers to the property of the intestinal mucosa that ensures adequate containment of undesirable luminal contents within the intestine while preserving the ability to absorb nutrients. The separation it provides between the body and the gut prevents the uncontrolled translocation of luminal contents into the body proper. Its role in protecting the mucosal tissues and circulatory system from exposure to pro-inflammatory molecules, such as microorganisms, toxins, and antigens is vital for the maintenance of health and well-being. Intestinal mucosal barrier dysfunction has been implicated in numerous health conditions such as: food allergies, microbial infections, irritable bowel syndrome, inflammatory bowel disease, celiac disease, metabolic syndrome, non-alcoholic fatty liver disease, diabetes, and septic shock.
An innate immune defect is a defect in the innate immune response that blunts the response to infection. These defects may occur in monocytes, neutrophils, natural killer cells, basophils, mast cells or complement proteins.
Carol Shoshkes Reiss, an American viral immunologist, was a professor at New York University's Department of Biology between 1991 and 2020. She is currently a professor emerita at New York University. Her research focused on the dynamic contest between the mouse immune system and virus replication during central nervous system infection. Reiss was editor-in-chief of the journal Viral Immunology (2000–2006) and is currently editor-in-chief of the journal DNA and Cell Biology (2012–present).

Carla V. Rothlin is an Argentinian immunologist. She is a professor of immunobiology at Yale University, where she holds the Dorys McConnell Duberg Professorship, and also serves as a professor of pharmacology. Rothlin is the co-leader of the Cancer Immunology Program at Yale Cancer Center and a Howard Hughes Medical Investigator Faculty Scholar.
References
- 1 2 3 "Jean Marshall". Dalhousie University. Retrieved 2019-03-26.
- 1 2 Marshall, Jean S.; Rowden, Geoffrey; Jawdat, Dunia M. (2006-08-01). "Mast Cells Have a Pivotal Role in TNF-Independent Lymph Node Hypertrophy and the Mobilization of Langerhans Cells in Response to Bacterial Peptidoglycan". The Journal of Immunology. 177 (3): 1755–1762. doi: 10.4049/jimmunol.177.3.1755 . ISSN 0022-1767. PMID 16849485.
- ↑ Bunce, Campbell; Bell, Eric B. (1997-02-17). "CD45RC Isoforms Define Two Types of CD4 Memory T Cells, One of which Depends on Persisting Antigen". The Journal of Experimental Medicine. 185 (4): 767–776. doi:10.1084/jem.185.4.767. ISSN 0022-1007. PMC 2196145 . PMID 9034154.
- ↑ "Dr. John Bienenstock | Canadian Medical Hall of Fame". 2014-08-19. Archived from the original on 2014-08-19. Retrieved 2019-03-26.
- ↑ King, Christine A.; Anderson, Robert; Marshall, Jean S. (August 2002). "Dengue Virus Selectively Induces Human Mast Cell Chemokine Production". Journal of Virology. 76 (16): 8408–8419. doi:10.1128/JVI.76.16.8408-8419.2002. ISSN 0022-538X. PMC 155122 . PMID 12134044.
- ↑ Marshall, Jean S.; Johnston, Brent; Leiva, Carlos A.; Howatt, Mackenzie A.; Haidl, Ian D.; Oldford, Sharon A. (2010-12-01). "A Critical Role for Mast Cells and Mast Cell-Derived IL-6 in TLR2-Mediated Inhibition of Tumor Growth". The Journal of Immunology. 185 (11): 7067–7076. doi: 10.4049/jimmunol.1001137 . ISSN 0022-1767. PMID 21041732.
- ↑ Rogers, Dakota; Vila-Leahey, Ava; Pessôa, Ana Clara; Oldford, Sharon; Marignani, Paola A.; Marshall, Jean S. (2018-08-15). "Ranitidine Inhibition of Breast Tumor Growth Is B Cell Dependent and Associated With an Enhanced Antitumor Antibody Response". Frontiers in Immunology. 9: 1894. doi: 10.3389/fimmu.2018.01894 . ISSN 1664-3224. PMC 6104125 . PMID 30158936.
- ↑ "Dal researcher wins Canada's top immunology award". Dalhousie News. Retrieved 2019-03-26.
- ↑ "Research Management Committee – AllerGen". Archived from the original on 2019-03-27. Retrieved 2019-03-26.
- 1 2 3 4 5 "User profiles for JS Marsha". Google Scholar. Retrieved January 19, 2019.