Related Research Articles

Nicotine is a naturally produced alkaloid in the nightshade family of plants and is widely used recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used for smoking cessation to relieve withdrawal symptoms. Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs), except at two nicotinic receptor subunits where it acts as a receptor antagonist.

Smoking cessation, usually called quitting smoking or stopping smoking, is the process of discontinuing tobacco smoking. Tobacco smoke contains nicotine, which is addictive and can cause dependence. As a result, nicotine withdrawal often makes the process of quitting difficult.
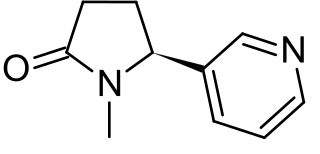
Cotinine is an alkaloid found in tobacco and is also the predominant metabolite of nicotine, typically used as a biomarker for exposure to tobacco smoke. Cotinine is currently being studied as a treatment for depression, post-traumatic stress disorder (PTSD), schizophrenia, Alzheimer's disease and Parkinson's disease. Cotinine was developed as an antidepressant as a fumaric acid salt, cotinine fumarate, to be sold under the brand name Scotine, but it was never marketed.

Nicotine replacement therapy (NRT) is a medically approved way to treat people with tobacco use disorder by taking nicotine through means other than tobacco. It is used to help with quitting smoking or stopping chewing tobacco. It increases the chance of quitting tobacco smoking by about 55%. Often it is used along with other behavioral techniques. NRT has also been used to treat ulcerative colitis. Types of NRT include the adhesive patch, chewing gum, lozenges, nose spray, and inhaler. The use of multiple types of NRT at a time may increase effectiveness.

Chain smoking is the practice of smoking several cigarettes in succession, sometimes using the ember of a finishing cigarette to light the next. The term chain smoker often also refers to a person who smokes relatively constantly, though not necessarily chaining each cigarette. The term applies primarily to cigarettes, although it can be used to describe incessant cigar and pipe smoking as well as vaping. It is a common indicator of addiction.

A nicotine patch is a transdermal patch that releases nicotine into the body through the skin. It is used in nicotine replacement therapy (NRT), a process for smoking cessation. Endorsed and approved by the U.S. Food and Drug Administration (FDA), it is considered one of the safer NRTs available for the treatment of tobacco use disorder.
Nicotine gum is a chewing gum containing the active ingredient nicotine polacrilex. It is a type of nicotine replacement therapy (NRT) used alone or in combination with other pharmacotherapy for smoking cessation and for quitting smokeless tobacco.
Nicorette is the brand name of a number of products for nicotine replacement therapy (NRT) that contain nicotine polacrilex. Developed in the late 1970s in Sweden by AB Leo in the form of a chewing gum, Nicorette was the first nicotine replacement product on the market.
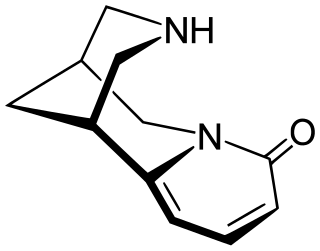
Cytisine, also known as baptitoxine, cytisinicline, or sophorine, is an alkaloid that occurs naturally in several plant genera, such as Laburnum and Cytisus of the family Fabaceae. It has been used medically to help with smoking cessation. It has been found effective in several randomized clinical trials, including in the United States and New Zealand, and is being investigated in additional trials in the United States and a non-inferiority trial in Australia in which it is being compared head-to-head with the smoking cessation aid varenicline. It has also been used entheogenically via mescalbeans by some Native American groups, historically in the Rio Grande Valley predating even peyote.

Varenicline, sold under the brand names Chantix and Champix among others, is a medication used for smoking cessation and for the treatment of dry eye syndrome. It is a nicotinic acetylcholine receptor partial agonist. When activated, this receptor releases dopamine in the nucleus accumbens, the brain's reward center, thereby reducing cravings and withdrawal symptoms with smoking cessation, although less pronounced than a full agonist.
NicVAX is an experimental conjugate vaccine intended to reduce or eliminate physical dependence to nicotine. According to the U.S. National Institute of Drug Abuse, NicVAX can potentially be used to inoculate against nicotine addiction. This proprietary vaccine is being developed by Nabi Biopharmaceuticals of Rockville, MD. with the support from the U.S. National Institute on Drug Abuse. NicVAX consists of the hapten 3'-aminomethylnicotine which has been conjugated (attached) to Pseudomonas aeruginosa exotoxin A.

Nicotine withdrawal is a group of symptoms that occur in the first few weeks after stopping or decreasing use of nicotine. Symptoms include intense cravings for nicotine, anger or irritability, anxiety, depression, impatience, trouble sleeping, restlessness, hunger, weight gain, and difficulty concentrating. Withdrawal symptoms make it harder to quit nicotine products, and most methods for quitting smoking involve reducing nicotine withdrawal. Quit smoking programs can make it easier to quit. Nicotine withdrawal is recognized in both the American Psychiatric Association Diagnostic and Statistical Manual (DSM) and the WHO International Classification of Diseases (ICD).
Tobacco harm reduction (THR) is a public health strategy to lower the health risks to individuals and wider society associated with using tobacco products. It is an example of the concept of harm reduction, a strategy for dealing with the use of drugs. Tobacco smoking is widely acknowledged as a leading cause of illness and death, and reducing smoking is vital to public health.

Nicotine dependence is a state of substance dependence on nicotine. It is a chronic, relapsing disease characterized by a compulsive craving to use the drug despite social consequences, loss of control over drug intake, and the emergence of withdrawal symptoms. Tolerance is another component of drug dependence. Nicotine dependence develops over time as an individual continues to use nicotine. While cigarettes are the most commonly used tobacco product, all forms of tobacco use—including smokeless tobacco and e-cigarette use—can cause dependence. Nicotine dependence is a serious public health problem because it leads to continued tobacco use and the associated negative health effects. Tobacco use is one of the leading preventable causes of death worldwide, causing more than 8 million deaths per year and killing half of its users who do not quit. Current smokers are estimated to die an average of 10 years earlier than non-smokers.
Nicotine Anonymous (NicA) is a twelve-step program founded in 1982 for people desiring to quit smoking and live free of nicotine. As of July 2017, there are over 700 face-to-face meetings in 32 countries worldwide with the majority of these meetings occurring in the United States, Iran, India, Canada, Brazil, the United Kingdom, Australia, Russia and in various online community and social media platforms.. NicA maintains that total abstinence from nicotine is necessary for recovery. NicA defines abstinence as “a state that begins when all use of nicotine ceases.
Elbert D. Glover is an American researcher and author in the field of tobacco addiction and smoking cessation. He retired as professor emeritus at the University of Maryland at College Park School of Public Health where he served as Chairperson of the Department of Behavioral and Community Health from 2005 to his retirement in 2015. He was an entrepreneur, editor, publisher, co-founder and principal owner of Health Behavior and Policy Review, and co-founder, owner, editor, and publisher of American Journal of Health Behavior and Tobacco Regulatory Science. Glover was the founder of the American Academy of Health Behavior and served as its first president from 1997 to 2001.
Murray Elias Jarvik was an American psychopharmacologist and academic who was among the first scientists to study d-lysergic acid, the precursor to LSD, and later became the co-inventor of the nicotine patch. He was a longtime professor emeritus at University of California-Los Angeles, where he taught as a professor of psychiatry and pharmacology for many years.

Dianicline (SSR-591,813) is a drug developed by Sanofi-Aventis which acts as a partial agonist at neural nicotinic acetylcholine receptors. It is subtype-selective, binding primarily to the α4β2 subtype. It is being developed as a medication for the treatment of nicotine dependence to assist in smoking cessation. Dianicline is very similar to the already marketed drug varenicline and it is unclear what advantages it will have over the older drug, although it may have an improved side effect profile. It has been through human trials up to Phase II, although results have not yet been reported. Drug development has been discontinued after reporting of unfavourable results during Phase III trials.
Schizophrenia and tobacco smoking have been historically associated. Smoking is known to harm the health of people with schizophrenia.
The Fagerström Test for Nicotine dependence is a standard instrument for assessing the intensity of addiction to nicotine. It evaluates the quantity of cigarette consumption, the compulsion to use, and dependence. In addition to this, the DSM-5 for tobacco use disorder can be used by physicians and nurse practitioners to make a diagnosis.
References
- 1 2 3 4 Rose, Jed. "Duke Medical Center Faculty Directory". Archived from the original on 19 September 2016. Retrieved 1 April 2016.
- ↑ "Method and apparatus for aiding in the reduction of incidence of tobacco smoking" . Retrieved 1 April 2016.
- ↑ "Rose Research Center". Archived from the original on 27 April 2016. Retrieved 1 April 2016.
- ↑ "Duke Center for Smoking Cessation" . Retrieved 1 April 2016.
- ↑ "Jed Eugene Rose Birth Record" . Retrieved 21 April 2016.[ permanent dead link ]
- ↑ "Murray E. Jarvik, Obituary" . Retrieved 4 April 2016.
- 1 2 Weber, Bruce (2008-05-13). "Murray Jarvik, 84, Whose Research Helped Lead to Nicotine Patch, Dies". The New York Times. Retrieved 21 April 2016.
- ↑ Ii, Thomas H. Maugh (2008-05-14). "Invention of the Nicotine Skin Patch". Los Angeles Times. Retrieved 4 April 2016.
- ↑ Ii, Thomas H. Maugh (2008-05-14). "Transdermal administration of nicotine". Los Angeles Times. Retrieved 4 April 2016.
- ↑ Ii, Thomas H. Maugh (2008-05-14). "Method and apparatus for aiding in the reduction of incidence of tobacco smoking". Los Angeles Times. Retrieved 4 April 2016.
- ↑ "Nicotine–mecamylamine treatment for smoking cessation: The role of pre-cessation therapy" . Retrieved 4 April 2016.
- ↑ Rose, JE; Behm, FM; Westman, EC; Levin, ED; Stein, RM; Ripka, GV (1994). "Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone". Clin Pharmacol Ther. 56 (1): 86–99. doi:10.1038/clpt.1994.105. PMID 8033499. S2CID 40192890.
- ↑ Rose, JE; Behm, FM; Westman, EC (2001). "Acute effects of nicotine and mecamylamine on tobacco withdrawal symptoms, cigarette reward and ad lib smoking". Pharmacol Biochem Behav. 68 (2): 187–97. doi:10.1016/s0091-3057(00)00465-2. PMID 11267622. S2CID 24866606.
- ↑ Rose, JE; Behm, FM; Westman, EC (1998). "Nicotine-mecamylamine treatment for smoking cessation: the role of pre-cessation therapy". Exp Clin Psychopharmacol. 6 (3): 331–43. doi:10.1037/1064-1297.6.3.331. PMID 9725117.
- ↑ "Refined cigarette smoke as a means to reduce nicotine intake" . Retrieved 4 April 2016.
- ↑ "The Health Consequences of Smoking: Nicotine Addiction: A Report of the Surgeon General". 1988. Retrieved 4 April 2016.
- ↑ "Sensory blockade of smoking satisfaction" . Retrieved 4 April 2016.
- ↑ Rose, JE; Behm, FM; Levin, ED (1993). "Role of nicotine dose and sensory cues in the regulation of smoke intake". Pharmacol Biochem Behav. 44 (4): 891–900. doi:10.1016/0091-3057(93)90021-k. PMID 8469698. S2CID 42902056.
- ↑ Westman, EC; Behm, FM; Rose, JE (1995). "Airway sensory replacement combined with nicotine replacement for smoking cessation. A randomized, placebo-controlled trial using a citric acid inhaler". Chest. 107 (5): 1358–64. doi:10.1378/chest.107.5.1358. PMID 7750331. S2CID 27147746.
- ↑ Rose, JE; Westman, EC; Behm, FM; Johnson, MP; Goldberg, JS (1999). "Blockade of smoking satisfaction using the peripheral nicotinic antagonist trimethaphan". Pharmacol Biochem Behav. 62 (1): 165–72. doi:10.1016/s0091-3057(98)00153-1. PMID 9972860. S2CID 2198708.
- ↑ "Better World Project Article". Archived from the original on 2016-03-09. Retrieved 2016-04-10.
- ↑ Rose, JE; Jarvik, ME; Rose, KD (May 1984). "Transdermal administration of nicotine". Drug and Alcohol Dependence. 13 (3): 209–213. doi:10.1016/0376-8716(84)90061-9. PMID 6734425.
- ↑ "Believe It or Not, Big Tobacco May Help Make Anti-Addiction Drugs". HuffPost . 2014-06-17. Retrieved 21 April 2016.
- ↑ Rose, Jed E.; Levin, Edward D.; Behm, Frederique M.; Westman, Eric C.; Stein, Roy M.; Lane, James D.; Ripka, Gail V. (1994). "Combined Agonist-Antagonist Treatment for Nicotine and Other Drug Dependencies". Neuropsychopharmacology. 11 (4): 281. doi: 10.1038/sj.npp.1380199 .
- ↑ Coe, JW; Brooks, PR; Vetelino, MG; et al. (May 2005). "Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation". J. Med. Chem. 48 (10): 3474–7. doi:10.1021/jm050069n. PMID 15887955. S2CID 83548212.
- ↑ "Ready to Quit Smoking?". Los Angeles Times . 16 February 2009.
- ↑ Yilmazel Ucar, E; Araz, O; Yilmaz, N; Akgun, M; Meral, M; Kaynar, H; Saglam, L (2014). "Effectiveness of pharmacologic therapies on smoking cessation success: three years results of a smoking cessation clinic". Multidiscip Respir Med. 9 (1): 9. doi: 10.1186/2049-6958-9-9 . PMC 3916028 . PMID 24495744.
- ↑ Rose, JE; Salley, A; Behm, FM; Bates, JE; Westman, EC (2010). "Reinforcing effects of nicotine and non-nicotine components of cigarette smoke". Psychopharmacology. 210 (1): 1–12. doi:10.1007/s00213-010-1810-2. PMC 4154143 . PMID 20358364.
- ↑ "Duke nicotine research conference examines tobacco addiction" . Retrieved 21 April 2016.
- ↑ Rose, J. E.; Mukhin, A. G.; Lokitz, S. J.; Turkington, T. G.; Herskovic, J.; Behm, F. M.; Garg, S.; Garg, P. K. (2010). "Kinetics of brain nicotine accumulation in dependent and nondependent smokers assessed with PET and cigarettes containing 11C-nicotine". Proceedings of the National Academy of Sciences. 107 (11): 5190–5195. Bibcode:2010PNAS..107.5190R. doi: 10.1073/pnas.0909184107 . PMC 2841893 . PMID 20212132.
- ↑ McKinney, DL; Gogova, M; Davies, BD; Ramakrishnan, V; Fisher, K; Carter, WH; Karnes, HT; Garnett, WR; Iyer, SS; Somani, AA; Kobal, G; Barr, WH (2012). "Evaluation of the effect of ammonia on nicotine pharmacokinetics using rapid arterial sampling". Nicotine Tob Res. 14 (5): 586–95. doi:10.1093/ntr/ntr257. PMID 22140146. S2CID 20624588.
- ↑ "Smokers Double Their Quit Rate By Wearing Nicotine Patch Before Stopping".
- ↑ "Nicotine Patch Before Quitting Doubles Success Rate".
- ↑ Rose, JE; Behm, FM (2013). "Adapting smoking cessation treatment according to initial response to precessation nicotine patch". Am J Psychiatry. 170 (8): 860–7. doi:10.1176/appi.ajp.2013.12070919. PMC 4562286 . PMID 23640009.
- ↑ Uhl, G. R.; Walther, D.; Musci, R.; Fisher, C.; Anthony, J. C.; Storr, C. L.; Behm, F. M.; Eaton, W. W.; Ialongo, N.; Rose, J. E. (2014). "Smoking quit success genotype score predicts quit success and distinct patterns of developmental involvement with common addictive substances". Molecular Psychiatry. 19 (1): 50–54. doi:10.1038/mp.2012.155. PMC 3922203 . PMID 23128154.
- ↑ Rose, JE; Behm, FM; Drgon, T; Johnson, C; Uhl, GR (2010). "Personalized smoking cessation: interactions between nicotine dose, dependence and quit-success genotype score". Mol. Med. 16 (7–8): 247–53. doi:10.2119/molmed.2009.00159. PMC 2896464 . PMID 20379614.
- ↑ Rose, JE; Herskovic, JE; Behm, FM; Westman, EC (2009). "Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment". Nicotine Tob Res. 11 (9): 1067–75. doi:10.1093/ntr/ntp103. PMID 19567826.
- ↑ Rose, JE; Behm, FM (2014). "Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm". Am J Psychiatry. 171 (11): 1199–205. doi:10.1176/appi.ajp.2014.13050595. PMC 4557205 . PMID 24934962.