Related Research Articles

Nature versus nurture is a long-standing debate in biology and society about the balance between two competing factors which determine fate: genetics (nature) and environment (nurture). The alliterative expression "nature and nurture" in English has been in use since at least the Elizabethan period and goes back to medieval French.
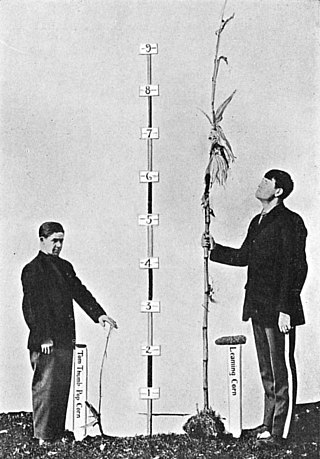
Heritability is a statistic used in the fields of breeding and genetics that estimates the degree of variation in a phenotypic trait in a population that is due to genetic variation between individuals in that population. The concept of heritability can be expressed in the form of the following question: "What is the proportion of the variation in a given trait within a population that is not explained by the environment or random chance?"

Human behaviour genetics is an interdisciplinary subfield of behaviour genetics that studies the role of genetic and environmental influences on human behaviour. Classically, human behavioural geneticists have studied the inheritance of behavioural traits. The field was originally focused on determining the importance of genetic influences on human behaviour. It has evolved to address more complex questions such as: how important are genetic and/or environmental influences on various human behavioural traits; to what extent do the same genetic and/or environmental influences impact the overlap between human behavioural traits; how do genetic and/or environmental influences on behaviour change across development; and what environmental factors moderate the importance of genetic effects on human behaviour. The field is interdisciplinary, and draws from genetics, psychology, and statistics. Most recently, the field has moved into the area of statistical genetics, with many behavioural geneticists also involved in efforts to identify the specific genes involved in human behaviour, and to understand how the effects associated with these genes changes across time, and in conjunction with the environment.
The candidate gene approach to conducting genetic association studies focuses on associations between genetic variation within pre-specified genes of interest, and phenotypes or disease states. This is in contrast to genome-wide association studies (GWAS), which is a hypothesis-free approach that scans the entire genome for associations between common genetic variants and traits of interest. Candidate genes are most often selected for study based on a priori knowledge of the gene's biological functional impact on the trait or disease in question. The rationale behind focusing on allelic variation in specific, biologically relevant regions of the genome is that certain alleles within a gene may directly impact the function of the gene in question and lead to variation in the phenotype or disease state being investigated. This approach often uses the case-control study design to try to answer the question, "Is one allele of a candidate gene more frequently seen in subjects with the disease than in subjects without the disease?" Candidate genes hypothesized to be associated with complex traits have generally not been replicated by subsequent GWASs or highly powered replication attempts. The failure of candidate gene studies to shed light on the specific genes underlying such traits has been ascribed to insufficient statistical power, low prior probability that scientists can correctly guess a specific allele within a specific gene that is related to a trait, poor methodological practices, and data dredging.

Gene–environment interaction is when two different genotypes respond to environmental variation in different ways. A norm of reaction is a graph that shows the relationship between genes and environmental factors when phenotypic differences are continuous. They can help illustrate GxE interactions. When the norm of reaction is not parallel, as shown in the figure below, there is a gene by environment interaction. This indicates that each genotype responds to environmental variation in a different way. Environmental variation can be physical, chemical, biological, behavior patterns or life events.
In mathematical or statistical modeling a threshold model is any model where a threshold value, or set of threshold values, is used to distinguish ranges of values where the behaviour predicted by the model varies in some important way. A particularly important instance arises in toxicology, where the model for the effect of a drug may be that there is zero effect for a dose below a critical or threshold value, while an effect of some significance exists above that value. Certain types of regression model may include threshold effects.

In genomics, a genome-wide association study, is an observational study of a genome-wide set of genetic variants in different individuals to see if any variant is associated with a trait. GWA studies typically focus on associations between single-nucleotide polymorphisms (SNPs) and traits like major human diseases, but can equally be applied to any other genetic variants and any other organisms.
In multivariate quantitative genetics, a genetic correlation is the proportion of variance that two traits share due to genetic causes, the correlation between the genetic influences on a trait and the genetic influences on a different trait estimating the degree of pleiotropy or causal overlap. A genetic correlation of 0 implies that the genetic effects on one trait are independent of the other, while a correlation of 1 implies that all of the genetic influences on the two traits are identical. The bivariate genetic correlation can be generalized to inferring genetic latent variable factors across > 2 traits using factor analysis. Genetic correlation models were introduced into behavioral genetics in the 1970s–1980s.

Behavioural genetics, also referred to as behaviour genetics, is a field of scientific research that uses genetic methods to investigate the nature and origins of individual differences in behaviour. While the name "behavioural genetics" connotes a focus on genetic influences, the field broadly investigates the extent to which genetic and environmental factors influence individual differences, and the development of research designs that can remove the confounding of genes and environment. Behavioural genetics was founded as a scientific discipline by Francis Galton in the late 19th century, only to be discredited through association with eugenics movements before and during World War II. In the latter half of the 20th century, the field saw renewed prominence with research on inheritance of behaviour and mental illness in humans, as well as research on genetically informative model organisms through selective breeding and crosses. In the late 20th and early 21st centuries, technological advances in molecular genetics made it possible to measure and modify the genome directly. This led to major advances in model organism research and in human studies, leading to new scientific discoveries.
The missing heritability problem is the fact that single genetic variations cannot account for much of the heritability of diseases, behaviors, and other phenotypes. This is a problem that has significant implications for medicine, since a person's susceptibility to disease may depend more on the combined effect of all the genes in the background than on the disease genes in the foreground, or the role of genes may have been severely overestimated.
The evolution of schizophrenia refers to the theory of natural selection working in favor of selecting traits that are characteristic of the disorder. Positive symptoms are features that are not present in healthy individuals but appear as a result of the disease process. These include visual and/or auditory hallucinations, delusions, paranoia, and major thought disorders. Negative symptoms refer to features that are normally present but are reduced or absent as a result of the disease process, including social withdrawal, apathy, anhedonia, alogia, and behavioral perseveration. Cognitive symptoms of schizophrenia involve disturbances in executive functions, working memory impairment, and inability to sustain attention.

Michael Edward "Mike" Goddard is a professorial fellow in animal genetics at the University of Melbourne, Australia.
Genome-wide complex trait analysis (GCTA) Genome-based restricted maximum likelihood (GREML) is a statistical method for variance component estimation in genetics which quantifies the total narrow-sense (additive) contribution to a trait's heritability of a particular subset of genetic variants. This is done by directly quantifying the chance genetic similarity of unrelated individuals and comparing it to their measured similarity on a trait; if two unrelated individuals are relatively similar genetically and also have similar trait measurements, then the measured genetics are likely to causally influence that trait, and the correlation can to some degree tell how much. This can be illustrated by plotting the squared pairwise trait differences between individuals against their estimated degree of relatedness. The GCTA framework can be applied in a variety of settings. For example, it can be used to examine changes in heritability over aging and development. It can also be extended to analyse bivariate genetic correlations between traits. There is an ongoing debate about whether GCTA generates reliable or stable estimates of heritability when used on current SNP data. The method is based on the outdated and false dichotomy of genes versus the environment. It also suffers from serious methodological weaknesses, such as susceptibility to population stratification.

In genetics, a polygenic score (PGS), also called a polygenic index (PGI), polygenic risk score (PRS), genetic risk score, or genome-wide score, is a number that summarizes the estimated effect of many genetic variants on an individual's phenotype, typically calculated as a weighted sum of trait-associated alleles. It reflects an individual's estimated genetic predisposition for a given trait and can be used as a predictor for that trait. In other words, it gives an estimate of how likely an individual is to have a given trait only based on genetics, without taking environmental factors into account. Polygenic scores are widely used in animal breeding and plant breeding due to their efficacy in improving livestock breeding and crops. In humans, polygenic scores are typically generated from genome-wide association study (GWAS) data.

Recent human evolution refers to evolutionary adaptation, sexual and natural selection, and genetic drift within Homo sapiens populations, since their separation and dispersal in the Middle Paleolithic about 50,000 years ago. Contrary to popular belief, not only are humans still evolving, their evolution since the dawn of agriculture is faster than ever before. It has been proposed that human culture acts as a selective force in human evolution and has accelerated it; however, this is disputed. With a sufficiently large data set and modern research methods, scientists can study the changes in the frequency of an allele occurring in a tiny subset of the population over a single lifetime, the shortest meaningful time scale in evolution. Comparing a given gene with that of other species enables geneticists to determine whether it is rapidly evolving in humans alone. For example, while human DNA is on average 98% identical to chimp DNA, the so-called Human Accelerated Region 1 (HAR1), involved in the development of the brain, is only 85% similar.

Complex traits, also known as quantitative traits, are traits that do not behave according to simple Mendelian inheritance laws. More specifically, their inheritance cannot be explained by the genetic segregation of a single gene. Such traits show a continuous range of variation and are influenced by both environmental and genetic factors. Compared to strictly Mendelian traits, complex traits are far more common, and because they can be hugely polygenic, they are studied using statistical techniques such as quantitative genetics and quantitative trait loci (QTL) mapping rather than classical genetics methods. Examples of complex traits include height, circadian rhythms, enzyme kinetics, and many diseases including diabetes and Parkinson's disease. One major goal of genetic research today is to better understand the molecular mechanisms through which genetic variants act to influence complex traits.
Naomi Ruth Wray is an Australian statistical geneticist at the University of Queensland, where she is a Professorial Research Fellow at the Institute for Molecular Bioscience and an Affiliate Professor in the Queensland Brain Institute. She is also a National Health and Medical Research Council (NHMRC) Principal Research Fellow and, along with Peter Visscher and Jian Yang, is one of the three executive team members of the NHMRC-funded Program in Complex Trait Genomics.
The Ruth Stephens Gani Medal is awarded annually by the Australian Academy of Science to recognise research in human genetics.
In statistical genetics, linkage disequilibrium score regression is a technique that aims to quantify the separate contributions of polygenic effects and various confounding factors, such as population stratification, based on summary statistics from genome-wide association studies (GWASs). The approach involves using regression analysis to examine the relationship between linkage disequilibrium scores and the test statistics of the single-nucleotide polymorphisms (SNPs) from the GWAS. Here, the "linkage disequilibrium score" for a SNP "is the sum of LD r2 measured with all other SNPs".
Personality traits are patterns of thoughts, feelings and behaviors that reflect the tendency to respond in certain ways under certain circumstances.
References
- ↑ "QBI researcher wins Australian Academy of Science award". Queensland Brain Institute. 24 November 2014. Retrieved 23 February 2019.
- ↑ "Australia's Life Scientist of the Year delves deep into the mysteries of heritability". Australian Research Council. 15 June 2018. Retrieved 23 February 2019.
- ↑ Slezak, Michael (18 October 2017). "Jenny Graves wins Australia's $250,000 prime minister's prize for science". The Guardian. Retrieved 23 February 2019.
- ↑ Koreis, Darius (8 October 2014). "No single gene makes you tall or short". Futurity. Retrieved 23 February 2019.
- ↑ Fleischfresser, Stephen (22 May 2018). "Stats point to natural selection drivers for weight, height, schizophrenia". Cosmos Magazine. Retrieved 23 February 2019.
- ↑ Mitchell-Whittington, Amy (15 January 2018). "Scientists find 'good' cholesterol could increase risk of eye disease". Brisbane Times. Retrieved 23 February 2019.