Related Research Articles

The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. It consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive element polonium (Po). The chemically uncharacterized synthetic element livermorium (Lv) is predicted to be a chalcogen as well. Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word khalkόs (χαλκός) principally meaning copper, and the Latinized Greek word genēs, meaning born or produced.

Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, and also has similarities to arsenic. It seldom occurs in its elemental state or as pure ore compounds in the Earth's crust. Selenium—from Greek selḗnē —was discovered in 1817 by Jöns Jacob Berzelius, who noted the similarity of the new element to the previously discovered tellurium.

Tellurium is a chemical element with the symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth.
A metalloid is a type of chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature of chemistry.
In chemistry, a nonmetal is a chemical element that generally lacks a predominance of metallic properties; they range from colorless gases to shiny and high melting temperature solids. The electrons in nonmetals behave differently from those in metals. With some exceptions, those in nonmetals are fixed in place, resulting in nonmetals usually being poor conductors of heat and electricity and brittle or crumbly when solid. The electrons in metals are generally free moving and this is why metals are good conductors and most are easily flattened into sheets and drawn into wires. Nonmetal atoms are moderately to highly electronegative; they tend to attract electrons in chemical reactions and to form acidic compounds.

Edward Divers FRS was a British experimental chemist who rose to prominence despite being visually impaired from young age. Between 1873 and 1899, Divers lived and worked in Japan and significantly contributed to the science and education of that country.

Tetrasulfur tetranitride is an inorganic compound with the formula S4N4. This gold-poppy coloured solid is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.

Lawesson's reagent, or LR, is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with P4S10.

Selenium dioxide is the chemical compound with the formula SeO2. This colorless solid is one of the most frequently encountered compounds of selenium.
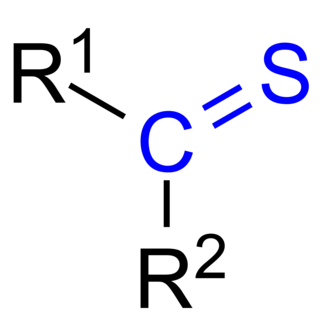
Thioketones (also known as thiones or thiocarbonyls) are organosulfur compounds related to conventional ketones in which the oxygen has been replaced by a sulfur. Instead of a structure of R2C=O, thioketones have the structure R2C=S, which is reflected by the prefix "thio-" in the name of the functional group. Unhindered alkylthioketones typically tend to form polymers or rings.

1,3,2,4-Dithiadiphosphetane 2,4-disulfides are a class of four-membered ring compounds which contain a P2S2 ring, many of these compounds are able to act as sources of the dithiophosphine ylides. The most well known example of this class of compound is Lawesson's reagent.
Organoselenium compounds are chemical compounds containing carbon-to-selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.
Selenium trioxide is the inorganic compound with the formula SeO3. It is white, hygroscopic solid. It is also an oxidizing agent and a Lewis acid. It is of academic interest as a precursor to Se(VI) compounds.
Organotellurium chemistry describes the synthesis and properties of chemical compounds containing a carbon-tellurium chemical bond. Organotellurium chemistry is a lightly studied area, in part because of the few applications.

Woollins' reagent is an organic compound containing phosphorus and selenium. Analogous to Lawesson's reagent, it is used mainly as a selenation reagent. It is named after Professor John Derek Woollins.
Phosphorus selenides are a relatively obscure group of compounds. There have been some studies of the phosphorus - selenium phase diagram and the glassy amorphous phases are reported. The compounds that have been reported are shown below. While some are similar to their phosphorus analogues, the phosphorus sulfides, there are some new forms, molecular P2Se5 and the polymeric catena-[P4Se4]x. There is also some doubt about the existence of molecular P4Se10.
Michael Bühl is a professor of Computational and Theoretical Chemistry in the School of Chemistry, University of St. Andrews. He has published work on the performance of various density functionals, modelling thermal and medium effects, transition-metal NMR of metalloenzymes, modelling of homogeneous catalysis, and molecular dynamics of transition metal complexes.

Nazira Karodia is a chemist, Professor of Science Education and Pro-Vice Chancellor for Regional Development at the University of Wolverhampton. She works on organic synthesis, green chemistry, heterocyclic compounds and science education.
References
- ↑ "Principal's Office". www.st-andrews.ac.uk. Retrieved 23 October 2018.
- ↑ "Prof J Derek Woollins Research Group". chemistry.st-and.ac.uk. Retrieved 26 January 2014.
- ↑ "European Academy of Sciences - List of Members". eurasc.org. Retrieved 3 April 2014.
- ↑ "Provost of St Leonard's College | University of St Andrews". www.st-andrews.ac.uk. Retrieved 6 May 2017.
- 1 2 Prof J Derek Woollins Research Group
- ↑ Prof J Derek Woollins Research Group
- ↑ Woollins, J.Derek; Rosenberg, Barnett (1983). "The detection of trace amounts of trans-Pt(NH3)2Cl2 in the presence of cis-Pt(NH3)2Cl2. A high performance liquid chromatographic application of kurnakow's test". Polyhedron. 2 (3): 175–178. doi:10.1016/S0277-5387(00)83954-6.
- ↑ Account Suspended
- ↑ "Archived copy". Archived from the original on 2 March 2016. Retrieved 18 February 2016.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Nordheider, Andreas; Chivers, Tristram; Schön, Oliver; Karaghiosoff, Konstantin; Athukorala Arachchige, Kasun S.; Slawin, Alexandra M. Z.; Woollins, J. Derek (2014). "Isolatable Organophosphorus(III)-Tellurium Heterocycles". Chemistry - A European Journal. 20 (3): 704–712. doi:10.1002/chem.201303884. hdl: 10023/5925 . PMID 24415362.
- ↑ Woollins, J. D.; Laitinen, R. S., eds. (2011). Selenium and Tellurium Chemistry From Small Molecules to Biomolecules and Materials. Springer Berlin. ISBN 9783642206986.
- ↑ Woollins, J. (2012). "How Not to Discover a New Reagent. The Evolution and Chemistry of Woollins' Reagent". Synlett. 23 (8): 1154–1169. doi:10.1055/s-0031-1290665. hdl: 10023/4577 .
- ↑ Hua, Guoxiong; Zhang, Qingzhi; McManus, Derek; Slawin, Alexandra M. Z.; Woollins, J. Derek (2006). "Novel non-aqueous Fe(III)/Fe(II) redox couple for the catalytic oxidation of hydrogen sulfide to sulfur by air". Dalton Transactions (9): 1147–1156. doi:10.1039/B513384J. PMID 16482350.
- ↑ Woollins, J. Derek (2010). "Phosphorus Heterocycles II Phosphorus Heterocycles II . Edited by Raj Kumar Bansal (University of Rajasthan, Jaipur, India)". Journal of the American Chemical Society. 132 (39): 13950. doi:10.1021/ja107961j. From the Series, Topics in Heterocyclic Chemistry, 21. Edited by R. R. Gupta . Springer-Verlag: Berlin, Heidelberg. 2010. xxii + 210 pp. $259. ISBN 978-3-642-12253-8
- ↑ Do, Truong Giang; Hupf, Emanuel; Nordheider, Andreas; Lork, Enno; Slawin, Alexandra M. Z.; Makarov, Sergey G.; Ketkov, Sergey Yu.; Mebs, Stefan; Woollins, J. Derek; Beckmann, Jens (2015). "Intramolecularly Group 15 Stabilized Aryltellurenyl Halides and Triflates". Organometallics. 34 (21): 5341–5360. doi:10.1021/acs.organomet.5b00813. hdl: 10023/9730 .
- ↑ RSC Sir Edward Frankland Fellowship Previous Winners
- ↑ RSC Main Group Chemistry Award Previous Winners
- ↑ Books by J. Derek Woollins (Author of Basic Atomic and Molecular Spectroscopy)
- ↑ J Derek Woollins – Research publications – University of St Andrews
- ↑ "Woollins". Google Scholar . Retrieved 25 April 2019.
- ↑ "Woollins". Google Scholar . Retrieved 19 June 2021.