Related Research Articles

In chemistry, an alkene is a hydrocarbon that contains a carbon–carbon double bond.

In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons from which one hydrogen atom has been removed are functional groups called hydrocarbyls. Hydrocarbons are generally colourless and hydrophobic with only weak odours. Because of their diverse molecular structures, it is difficult to generalize further. Most anthropogenic emissions of hydrocarbons are from the burning of fossil fuels including fuel production and combustion. Natural sources of hydrocarbons such as ethylene, isoprene, and monoterpenes come from the emissions of vegetation.
Sharpless asymmetric dihydroxylation is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol. The reaction has been applied to alkenes of virtually every substitution, often high enantioselectivities are realized. Asymmetric dihydroxylation reactions are also highly site selective, providing products derived from reaction of the most electron-rich double bond in the substrate.
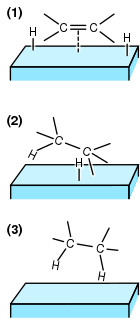
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.

An epoxide is a cyclic ether with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.
Halogenation is a chemical reaction that involves the addition of one or more halogens to a compound or material. The pathway and stoichiometry of halogenation depends on the structural features and functional groups of the organic substrate, as well as on the specific halogen. Inorganic compounds such as metals also undergo halogenation.

The Jacobsen epoxidation, sometimes also referred to as Jacobsen-Katsuki epoxidation is a chemical reaction which allows enantioselective epoxidation of unfunctionalized alkyl- and aryl- substituted alkenes. It is complementary to the Sharpless epoxidation (used to form epoxides from the double bond in allylic alcohols). The Jacobsen epoxidation gains its stereoselectivity from a C2 symmetric manganese(III) salen-like ligand, which is used in catalytic amounts. The manganese atom transfers an oxygen atom from chlorine bleach or similar oxidant. The reaction is named after its inventor, Eric Jacobsen, and sometimes also including Tsutomu Katsuki. Chiral-directing catalysts are useful to organic chemists trying to control the stereochemistry of biologically active compounds and develop enantiopure drugs.

The Shi epoxidation is a chemical reaction described as the asymmetric epoxidation of alkenes with oxone and a fructose-derived catalyst (1). This reaction is thought to proceed via a dioxirane intermediate, generated from the catalyst ketone by oxone. The addition of the sulfate group by the oxone facilitates the formation of the dioxirane by acting as a good leaving group during ring closure. It is notable for its use of a non-metal catalyst and represents an early example of organocatalysis. The reaction was first discovered by Yian Shi of Colorado State University in 1996.
Carbon–hydrogen bond functionalization is a type of reaction in which a carbon–hydrogen bond is cleaved and replaced with a carbon–X bond. The term usually implies that a transition metal is involved in the C-H cleavage process. Reactions classified by the term typically involve the hydrocarbon first to react with a metal catalyst to create an organometallic complex in which the hydrocarbon is coordinated to the inner-sphere of a metal, either via an intermediate "alkane or arene complex" or as a transition state leading to a "M−C" intermediate. The intermediate of this first step can then undergo subsequent reactions to produce the functionalized product. Important to this definition is the requirement that during the C–H cleavage event, the hydrocarbyl species remains associated in the inner-sphere and under the influence of "M".
Desulfatibacillum alkenivorans AK-01 is a specific strain of Desulfatibacillum alkenivorans.

Jacobsen's catalyst is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a coordination compound of manganese and a salen-type ligand. It is used as an asymmetric catalyst in the Jacobsen epoxidation, which is renowned for its ability to enantioselectively transform prochiral alkenes into epoxides. Before its development, catalysts for the asymmetric epoxidation of alkenes required the substrate to have a directing functional group, such as an alcohol as seen in the Sharpless epoxidation. This compound has two enantiomers, which give the appropriate epoxide product from the alkene starting material.
Catalytic oxidation are processes that oxidize compounds using catalysts. Common applications involve oxidation of organic compounds by the oxygen in air. Such processes are conducted on a large scale for the remediation of pollutants, production of valuable chemicals, and the production of energy. In petrochemistry, high-value intermediates such as carboxylic acids, aldehydes, ketones, epoxides, and alcohols are obtained by partial oxidation of alkanes and alkenes with dioxygen. These intermediates are essential to the production of consumer goods. Partial oxidation presents two challenges. The first is that the most favored reaction between oxygen and hydrocarbons is combustion. The second challenge is the considerable difficulty to activate dioxygen, viz. the splitting of the molecule into its constituent atoms, which has an energy barrier of 498 kJ/mol. The usual strategy to activate oxygen in a controlled manner is to use molecular hydrogen or carbon monoxide as sacrificial reductants in presence of a heterogeneous catalyst, such that the activation barrier is lowered to < 10 kJ/mol and hence milder reaction conditions are required.
Oxidation with dioxiranes refers to the introduction of oxygen into organic molecules through the action of a dioxirane. Dioxiranes are well known for their oxidation of alkenes to epoxides; however, they are also able to oxidize other unsaturated functionality, heteroatoms, and alkane C-H bonds.
A transition metal oxo complex is a coordination complex containing an oxo ligand. Formally O2-, an oxo ligand can be bound to one or more metal centers, i.e. it can exist as a terminal or (most commonly) as bridging ligands (Fig. 1). Oxo ligands stabilize high oxidation states of a metal.

An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon. In their largest application, oxaziridines are intermediates in the industrial production of hydrazine. Oxaziridine derivatives are also used as specialized reagents in organic chemistry for a variety of oxidations, including alpha hydroxylation of enolates, epoxidation and aziridination of olefins, and other heteroatom transfer reactions. Oxaziridines also serve as precursors to amides and participate in [3+2] cycloadditions with various heterocumulenes to form substituted five-membered heterocycles. Chiral oxaziridine derivatives effect asymmetric oxygen transfer to prochiral enolates as well as other substrates. Some oxaziridines also have the property of a high barrier to inversion of the nitrogen, allowing for the possibility of chirality at the nitrogen center.
A tosylhydrazone in organic chemistry is a functional group with the general structure RR'C=N-NH-Ts where Ts is a tosyl group. Organic compounds having this functional group can be accessed by reaction of an aldehyde or ketone with tosylhydrazine.
The White–Chen catalyst is an Iron-based coordination complex named after Professor M. Christina White and her graduate student Mark S. Chen. The catalyst is used along with hydrogen peroxide and acetic acid additive to oxidize aliphatic sp3 C-H bonds in organic synthesis. The catalyst is the first to allow for preparative and predictable aliphatic C–H oxidations over a broad range of organic substrates. Oxidations with the catalyst have proven to be remarkably predictable based on sterics, electronics, and stereoelectronics allowing for aliphatic C–H bonds to be thought of as a functional group in the streamlining of organic synthesis.

Allyl glycidyl ether is an organic compound used in adhesives and sealants and as a monomer for polymerization reactions. It is formally the condensation product of allyl alcohol and glycidol via an ether linkage. Because it contains both an alkene and an epoxide group, either group can be reacted selectively to yield a product where the other functional group remains intact for future reactions.
Cobalt(II)–porphyrin catalysis is a process in which a Co(II) porphyrin complex acts as a catalyst, inducing and accelerating a chemical reaction.
Daniel Mansuy is a French researcher and chemist born in 1945 in Châteauroux (Indre), a member of the French Academy of Sciences.
References
- ↑ Parker, Hilary (December 21, 2009). "Princeton University - Celebrate Princeton Invention: John Groves". Princeton University. Retrieved September 25, 2011.
- ↑ "John T. Groves". Princeton University. Retrieved September 25, 2011.
- ↑ "People — Center for Catalytic Hydrocarbon Functionalization". University of Virginia College and Graduate School of Arts and Sciences. Retrieved September 25, 2011.