Related Research Articles
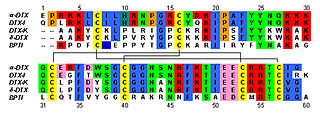
Dendrotoxins are a class of presynaptic neurotoxins produced by mamba snakes (Dendroaspis) that block particular subtypes of voltage-gated potassium channels in neurons, thereby enhancing the release of acetylcholine at neuromuscular junctions. Because of their high potency and selectivity for potassium channels, dendrotoxins have proven to be extremely useful as pharmacological tools for studying the structure and function of these ion channel proteins.

Anemonia sulcata, or Mediterranean snakelocks sea anemone, is a species of sea anemone in the family Actiniidae from the Mediterranean Sea. Whether A. sulcata should be recognized as a synonym of A. viridis remains a matter of dispute.

Sea anemone neurotoxin is the name given to neurotoxins produced by sea anemones with related structure and function. Sea anemone neurotoxins can be divided in two functional groups that either specifically target the sodium channel or the potassium channel.
Mast cell degranulating (MCD) peptide is a cationic 22-amino acid residue peptide, which is a component of the venom of the bumblebee. At low concentrations, MCD peptide can stimulate mast cell degranulation. At higher concentrations, it has anti-inflammatory properties. In addition, it is a potent blocker of voltage-sensitive potassium channels.
AETX refers to a group of polypeptide neurotoxins isolated from the sea anemone Anemonia erythraea that target ion channels, altering their function. Four subtypes have been identified: AETX I, II, III and K, which vary in their structure and target.
Hemitoxin (HTX; α-KTx6.15) is a 35-mer basic peptide from the venom of the Iranian scorpion Hemiscorpius lepturus, which reversibly blocks Kv1.1, Kv1.2 and Kv1.3voltage-gated K+ channels.
Parabutoxin (PBTx) is a Shaker-related voltage-gated K+ channel (Kvα1) inhibitor purified from different Parabuthus scorpion species found in southern Africa. It occurs in different forms: parabutoxin 1 (PBTx1), parabutoxin 2 (PBTx2), parabutoxin 3 (PBTx3) and parabutoxin (PBTx10). The different variants have different affinities towards Kvα1 channels.
Anuroctoxin is a peptide from the venom of the Mexican scorpion Anuroctonus phaiodactylus. This neurotoxin belongs to the alpha family of potassium channel acting peptides. It is a high-affinity blocker of Kv1.3 channels.

Pi3 toxin is a purified peptide derivative of the Pandinus imperator scorpion venom. It is a potent blocker of voltage-gated potassium channel, Kv1.3 and is closely related to another peptide found in the venom, Pi2.
Calitoxin, also known as CLX, is a sea anemone neurotoxin produced by the sea anemone Calliactis parasitica. It targets crabs and octopuses, among other invertebrates. Two isoforms have been identified, both of which are formed from precursors stored in the stinging cells of the anemone. Once the toxin is activated and released, it causes paralysis by increasing neurotransmitter release at invertebrate neuromuscular junctions. Along with several other toxins derived from anemones, CLX is useful in ion channel research. Certain structural aspects of calitoxin are dissimilar from sea anemone toxins that also target the sodium ion channels. Other toxins resembling calitoxin function in completely different ways.
BgK is a neurotoxin found within secretions of the sea anemone Bunodosomagranulifera which blocks voltage-gated potassium channels, thus inhibiting neuronal repolarization.
Blood-depressing substance-1 (BDS-1), also known as kappa-actitoxin-Avd4a, is a polypeptide found in the venom of the snakelocks anemone Anemonia sulcata. BDS-1 is a neurotoxin that modulates voltage-dependent potassium channels, in particular Kv3-family channels, as well as certain sodium channels. This polypeptide belongs to the sea anemone type 3 toxin peptide family.
HsTx1 is a toxin from the venom of the scorpion Heterometrus spinifer. HsTx1 is a very potent inhibitor of the rat Kv1.3 voltage-gated potassium channel.
Spinoxin is a 34-residue peptide neurotoxin isolated from the venom of the Malaysian black scorpion Heterometrus spinifer. It is part of the α-KTx6 subfamily and exerts its effects by inhibiting voltage-gated potassium channels, specifically Kv1.2 and Kv1.3.
SHTX is a toxin derived from the sea anemone Stichodactyla haddoni; there are four different subtypes, SHTX I, II, III and IV. SHTX I, II and III can paralyze crabs by acting on potassium channels, while SHTX IV works on sodium channels, and is lethal to crabs.
Kalicludine (AsKC) is a blocker of the voltage-dependent potassium channel Kv1.2 found in the snakeslocks anemone Anemonia viridis, which it uses to paralyse prey.
ATX-II, also known as neurotoxin 2, Av2, Anemonia viridis toxin 2 or δ-AITX-Avd1c, is a neurotoxin derived from the venom of the sea anemone Anemonia sulcata. ATX-II slows down the inactivation of different voltage-gated sodium channels, including Nav1.1 and Nav1.2, thus prolonging action potentials.
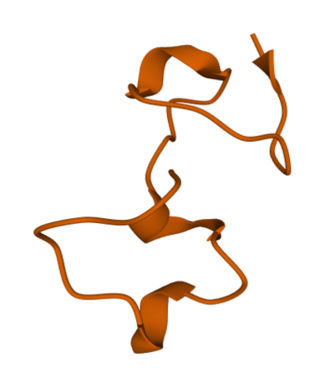
BcsTx3, also known as Kappa-actitoxin-Bsc4a, is a neurotoxin that blocks voltage-gated potassium channel (Kv1/KCNA). It is purified from the venom of Bunodosoma caissarum.
AsKC11 is a toxin found in the venom of the sea anemone, Anemonia sulcata. This toxin is part of the Kunitz peptide family and has been shown to be an activator of G protein-coupled inwardly-rectifying potassium (GIRK) channels 1/2, involved in the regulation of cellular excitability.
Kunitz-type serine protease inhibitor APEKTx1 is a peptide toxin derived from the sea anemone Anthopleura elegantissima. This toxin has a dual function, acting both as a serine protease inhibitor and as a selective and potent pore blocker of Kv1.1, a shaker related voltage-gated potassium channel.
References
- 1 2 3 4 5 6 7 8 9 Schweitz, H.; Bruhn, T. (20 October 1995). "Kalicludines and kaliseptine. Two different classes of sea anemone toxins for voltage sensitive K+ channels". The Journal of Biological Chemistry. 270 (42): 25121–25126. doi: 10.1074/jbc.270.42.25121 . PMID 7559645.
- 1 2 Oliveira, J.S.; Fuentes-Silva, D. (15 September 2012). "Development of a rational nomenclature for naming peptide and protein toxins from sea anemones". Toxicon. 60 (4): 539–550. Bibcode:2012Txcn...60..539O. doi:10.1016/j.toxicon.2012.05.020. PMID 22683676.
- ↑ "UniProtKB - Q9TWG1 (TXT1B_ANESU)". UniProt. Retrieved 7 October 2015.
- 1 2 3 4 Minagawa, S.; Ishida, M. (1 May 1998). "Primary structure of a potassium channel toxin from the sea anemone Actinia equina". FEBS Letters. 427 (1): 149–151. Bibcode:1998FEBSL.427..149M. doi: 10.1016/s0014-5793(98)00403-7 . PMID 9613617.
- ↑ Moran, Y.; Genikhovich, G. (7 April 2012). "Neurotoxin localization to ectodermal gland cells uncovers an alternative mechanism of venom delivery in sea anemones". Proceedings of the Royal Society B. 279 (1732): 1351–1358. doi:10.1098/rspb.2011.1731. PMC 3282367 . PMID 22048953.
- ↑ Honma, T.; Shiomi, K. (January 2006). "Peptide toxins in sea anemones: structural and functional aspects". Marine Biotechnology. 8 (1): 1–10. Bibcode:2006MarBt...8....1H. doi:10.1007/s10126-005-5093-2. PMC 4271777 . PMID 16372161.
- ↑ Pennington, M.W.; Mahnir, V.M. (1996). "An essential binding surface for ShK toxin interaction with rat brain potassium channels". Biochemistry. 35 (51): 16407–16411. doi:10.1021/bi962463g. PMID 8987971.
- ↑ Hurst, R.S.; Busch, A.E. (October 1991). "Identification of amino acid residues involved in dendrotoxin block of rat voltage-dependent potassium channels". Molecular Pharmacology. 40 (4): 572–576. PMID 1921987.
- ↑ Imredy, J.P.; MacKinnon, R. (March 2000). "Energetic and structural interactions between delta-dendrotoxin and a voltage-gated potassium channel". Journal of Molecular Biology. 296 (5): 1283–1294. doi:10.1006/jmbi.2000.3522. PMID 10698633.
- ↑ Isenberg, G.; Ravens, U. (December 1984). "The effects of the Anemonia sulcata toxin (ATX II) on membrane currents of isolated mammalian myocytes". The Journal of Physiology. 357: 127–149. doi:10.1113/jphysiol.1984.sp015493. PMC 1193251 . PMID 6150992.
- ↑ Alsen, C.; Béress, L. (October 1976). "The action of a toxin from the sea anemone Anemonia sulcata upon Mammalian heart muscles". Naunyn-Schmiedeberg's Archives of Pharmacology. 295 (1): 55–62. doi:10.1007/bf00509773. PMID 12483. S2CID 6807646.
- ↑ Tazieff-Depierre, F.; Choucavy, M. (27 September 1976). "Pharmacologic properties of the toxins isolated from the sea anemone (Anemonia sulcata)". Comptes Rendus de l'Académie des Sciences, Série D. 283 (6): 699–702. PMID 186216.
- 1 2 Hoey, A.; Harrison, S.M. (December 1994). "Effects of the Anemonia sulcata toxin (ATX II) on intracellular sodium and contractility in rat and guinea-pig myocardium". Parmacology & Toxicology. 75 (6): 356–365. doi:10.1111/j.1600-0773.1994.tb00375.x. PMID 7899257.
- ↑ Maretić, Z.; Russell, F.E. (July 1983). "Stings by the sea anemone Anemonia sulcata in the Adriatic Sea". The American Journal of Tropical Medicine and Hygiene. 32 (4): 891–896. doi:10.4269/ajtmh.1983.32.891. PMID 6136192.
- ↑ Abody, Z.; Klein-Kremer, A. (October 2006). "Anemonia sulcata sting". Harefuah. 145 (10): 736–737. PMID 17111708.
- ↑ Rosson, C.L.; Tolle, S.W. (January 1989). "Management of marine stings and scrapes". The Western Journal of Medicine. 150 (1): 97–100. PMC 1026320 . PMID 2567557.