
Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers in the parasympathetic nervous system.

A muscarinic agonist is an agent that activates the activity of the muscarinic acetylcholine receptor. The muscarinic receptor has different subtypes, labelled M1-M5, allowing for further differentiation.
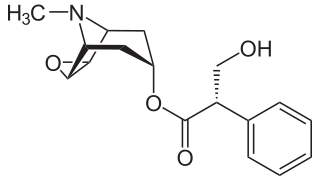
A muscarinic receptor antagonist (MRA) is a type of anticholinergic agent that blocks the activity of the muscarinic acetylcholine receptor. The muscarinic receptor is a protein involved in the transmission of signals through certain parts of the nervous system, and muscarinic receptor antagonists work to prevent this transmission from occurring. Notably, muscarinic antagonists reduce the activation of the parasympathetic nervous system. The normal function of the parasympathetic system is often summarised as "rest-and-digest", and includes slowing of the heart, an increased rate of digestion, narrowing of the airways, promotion of urination, and sexual arousal. Muscarinic antagonists counter this parasympathetic "rest-and-digest" response, and also work elsewhere in both the central and peripheral nervous systems.

The muscarinic acetylcholine receptor M1, also known as the cholinergic receptor, muscarinic 1, is a muscarinic receptor that in humans is encoded by the CHRM1 gene. It is localized to 11q13.

The muscarinic acetylcholine receptor M4, also known as the cholinergic receptor, muscarinic 4 (CHRM4), is a protein that, in humans, is encoded by the CHRM4 gene.
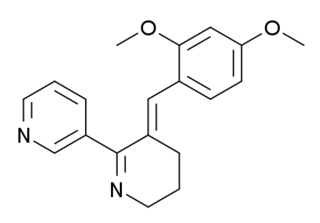
GTS-21 is an investigational drug that has been studied for its potential therapeutic uses, particularly in the treatment of neurodegenerative diseases and psychiatric disorders.
An H3 receptor antagonist is a type of antihistaminic drug used to block the action of histamine at H3 receptors.

Xanomeline is a small molecule muscarinic acetylcholine receptor agonist that was first synthesized in a collaboration between Eli Lilly and Novo Nordisk as an investigational therapeutic being studied for the treatment of central nervous system disorders.

Vedaclidine (INN, codenamed LY-297,802, NNC 11-1053) is an experimental analgesic drug which acts as a mixed agonist–antagonist at muscarinic acetylcholine receptors, being a potent and selective agonist for the M1 and M4 subtypes, yet an antagonist at the M2, M3 and M5 subtypes. It is orally active and an effective analgesic over 3× the potency of morphine, with side effects such as salivation and tremor only occurring at many times the effective analgesic dose. Human trials showed little potential for development of dependence or abuse, and research is continuing into possible clinical application in the treatment of neuropathic pain and cancer pain relief.

Pimavanserin, sold under the brand name Nuplazid, is an atypical antipsychotic which is approved for the treatment of Parkinson's disease psychosis and is also being studied for the treatment of Alzheimer's disease psychosis, schizophrenia, agitation, and major depressive disorder. Unlike other antipsychotics, pimavanserin is not a dopamine receptor antagonist.

Pomaglumetad (LY-404,039) is an amino acid analog drug that acts as a highly selective agonist for the metabotropic glutamate receptor group II subtypes mGluR2 and mGluR3. Pharmacological research has focused on its potential antipsychotic and anxiolytic effects. Pomaglumetad is intended as a treatment for schizophrenia and other psychotic and anxiety disorders by modulating glutamatergic activity and reducing presynaptic release of glutamate at synapses in limbic and forebrain areas relevant to these disorders. Human studies investigating therapeutic use of pomaglumetad have focused on the prodrug LY-2140023, a methionine amide of pomaglumetad (also called pomaglumetad methionil) since pomaglumetad exhibits low oral absorption and bioavailability in humans.

Alvameline (Lu 25-109) is a M1 receptor agonist and M2/M3 receptor antagonist that was under investigation for the treatment of Alzheimer's disease, but produced poor results in clinical trials and was subsequently discontinued.

Milameline is a non-selective muscarinic acetylcholine receptor partial agonist with cognition-acting properties that was being investigated for the treatment of Alzheimer's disease, but produced poor results in clinical trials and was subsequently discontinued.

N-Desmethylclozapine (NDMC), or norclozapine, is a major active metabolite of the atypical antipsychotic drug clozapine. Unlike clozapine, it possesses intrinsic activity at the D2/D3 receptors, and acts as a weak partial agonist at these sites similarly to aripiprazole and bifeprunox. Notably, NDMC has also been shown to act as a potent and efficacious agonist at the M1 and δ-opioid receptors, unlike clozapine as well. It was hypothesized that on account of these unique actions, NDMC might underlie the clinical superiority of clozapine over other antipsychotics. However, clinical trials found NMDC itself ineffective in the treatment of schizophrenia. This may be because it possesses relatively low D2/D3 occupancy compared to 5-HT2 (<15% versus 64–79% at a dose of 10–60 mg/kg s.c. in animal studies). Albeit not useful in the treatment of positive symptoms on its own, it cannot be ruled out that NDMC may contribute to the efficacy of clozapine on cognitive and/or negative symptoms.
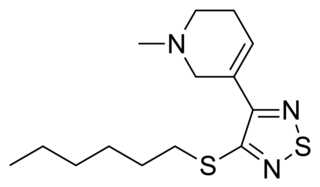
Tazomeline (LY-287,041) is a drug which acts as a non-selective muscarinic acetylcholine receptor agonist. It was in clinical trials for the treatment of cognitive dysfunction such as that seen in Alzheimer's disease and schizophrenia, but development was apparently scrapped for unknown reasons. Another of the patented uses is for the treatment of "severe painful conditions".
CI-1017 is a muscarinic acetylcholine receptor agonist which is selective for and is approximately equipotent at the M1 and M4 receptors, with 20-30-fold lower affinity for the M2, M3, and M5 subtypes It is the (R)-enantiomer of the racemic compound PD-142,505.
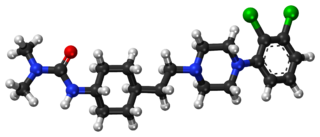
Cariprazine, sold under the brand names Vraylar,Reagila and Symvenu among others, is an atypical antipsychotic originated by Gedeon Richter, which is used in the treatment of schizophrenia, bipolar mania, bipolar depression, and major depressive disorder. It acts primarily as a D3 and D2 receptor partial agonist, with a preference for the D3 receptor. Cariprazine is also a partial agonist at the serotonin 5-HT1A receptor and acts as an antagonist at 5-HT2B and 5-HT2A receptors, with high selectivity for the D3 receptor. It is taken by mouth.

Brexpiprazole, sold under the brand name Rexulti among others, is a medication used for the treatment of major depressive disorder, schizophrenia, and agitation associated with dementia due to Alzheimer's disease. It is an atypical antipsychotic.
Emraclidine is an investigational antipsychotic for the treatment of both schizophrenia and Alzheimer’s disease psychosis developed by Cerevel Therapeutics. As of February 2023, it is in phase II of clinical trial. Emraclidine is a positive allosteric modulator that selectively targets the muscarinic acetylcholine receptor M4 subtype. The M4 receptor subtype is expressed in the striatum of the brain, which plays a key role in regulating acetylcholine and dopamine levels. An imbalance of these neurotransmitters has been linked to psychotic symptoms in schizophrenia. Unlike other muscarinic receptors, M4 receptor subtypes are selectively expressed in the striatum and activation of these receptors has been shown to indirectly regulate dopamine levels without blocking D2/D3 receptors, which may lead to unwanted motor side effects seen in current antipsychotics.















