Related Research Articles
Degenerative disease is the result of a continuous process based on degenerative cell changes, affecting tissues or organs, which will increasingly deteriorate over time.
Neuroprotection refers to the relative preservation of neuronal structure and/or function. In the case of an ongoing insult the relative preservation of neuronal integrity implies a reduction in the rate of neuronal loss over time, which can be expressed as a differential equation. It is a widely explored treatment option for many central nervous system (CNS) disorders including neurodegenerative diseases, stroke, traumatic brain injury, spinal cord injury, and acute management of neurotoxin consumption. Neuroprotection aims to prevent or slow disease progression and secondary injuries by halting or at least slowing the loss of neurons. Despite differences in symptoms or injuries associated with CNS disorders, many of the mechanisms behind neurodegeneration are the same. Common mechanisms of neuronal injury include decreased delivery of oxygen and glucose to the brain, energy failure, increased levels in oxidative stress, mitochondrial dysfunction, excitotoxicity, inflammatory changes, iron accumulation, and protein aggregation. Of these mechanisms, neuroprotective treatments often target oxidative stress and excitotoxicity—both of which are highly associated with CNS disorders. Not only can oxidative stress and excitotoxicity trigger neuron cell death but when combined they have synergistic effects that cause even more degradation than on their own. Thus limiting excitotoxicity and oxidative stress is a very important aspect of neuroprotection. Common neuroprotective treatments are glutamate antagonists and antioxidants, which aim to limit excitotoxicity and oxidative stress respectively.

In genetics, a silencer is a DNA sequence capable of binding transcription regulation factors, called repressors. DNA contains genes and provides the template to produce messenger RNA (mRNA). That mRNA is then translated into proteins. When a repressor protein binds to the silencer region of DNA, RNA polymerase is prevented from transcribing the DNA sequence into RNA. With transcription blocked, the translation of RNA into proteins is impossible. Thus, silencers prevent genes from being expressed as proteins.
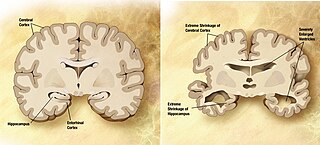
A neurodegenerative disease is caused by the progressive loss of structure or function of neurons, in the process known as neurodegeneration. Such neuronal damage may ultimately involve cell death. Neurodegenerative diseases include amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, Alzheimer's disease, Huntington's disease, multiple system atrophy, and prion diseases. Neurodegeneration can be found in the brain at many different levels of neuronal circuitry, ranging from molecular to systemic. Because there is no known way to reverse the progressive degeneration of neurons, these diseases are considered to be incurable; however research has shown that the two major contributing factors to neurodegeneration are oxidative stress and inflammation. Biomedical research has revealed many similarities between these diseases at the subcellular level, including atypical protein assemblies and induced cell death. These similarities suggest that therapeutic advances against one neurodegenerative disease might ameliorate other diseases as well.

RE1-Silencing Transcription factor (REST), also known as Neuron-Restrictive Silencer Factor (NRSF), is a protein which in humans is encoded by the REST gene, and acts as a transcriptional repressor. REST is expressly involved in the repression of neural genes in non-neuronal cells. Many genetic disorders have been tied to alterations in the REST expression pattern, including colon and small-cell lung carcinomas found with truncated versions of REST. In addition to these cancers, defects in REST have also been attributed a role in Huntington Disease, neuroblastomas, and the effects of epileptic seizures and ischemia.

Ann Martin Graybiel is an Institute Professor and a faculty member in the Department of Brain and Cognitive Sciences at the Massachusetts Institute of Technology. She is also an investigator at the McGovern Institute for Brain Research. She is an expert on the basal ganglia and the neurophysiology of habit formation, implicit learning, and her work is relevant to Parkinson's disease, Huntington's disease, obsessive–compulsive disorder, substance abuse and other disorders that affect the basal ganglia.
Bagadilico, Basal Ganglia Disorders Linnaeus Consortium, is a research group in Lund, Sweden, and a Linnaeus environment, supported by the Swedish Research Council. The group comprises about 120 researchers at either Lund University or Lund University Hospital.

The UCL Queen Square Institute of Neurology is an institute within the Faculty of Brain Sciences of University College London (UCL) and is located in London, United Kingdom. Together with the National Hospital for Neurology and Neurosurgery, an adjacent facility with which it cooperates closely, the institute forms a major centre for teaching, training and research in neurology and allied clinical and basic neurosciences.

The Illawarra Health and Medical Research Institute (IHMRI) is an independent health and medical research institute based in Wollongong, New South Wales.
Maria Grazia Spillantini, is Professor of Molecular Neurology in the Department of Clinical Neurosciences at the University of Cambridge. She is most noted for identifying the protein alpha-synuclein as the major component of Lewy bodies, the characteristic protein deposit found in the brain in Parkinson's disease and dementia with Lewy bodies. She has also identified mutations in the MAPT gene as a heritable cause for frontotemporal dementia.
Epigenetic regulation of neurogenesis is the role that epigenetics plays in the regulation of neurogenesis.

Giovanna Rachele Mallucci is van Geest Professor of Clinical Neurosciences at the University of Cambridge in England and associate director of the UK Dementia Research Institute at the University of Cambridge. She is a specialist in neurodegenerative diseases.
Dr. Natalie Matosin is an Australian scientist known for research into the impacts of stress and its role in mental illness. Matosin's research has been published in prestigious academic journals, as well as on The Conversation. Matosin spoke at TEDx Hamburg in June 2017 and is the 2021 Al & Val Rosenstrauss Fellow. She was previously a National Health and Medical Research Council CJ Martin Early Career Research Fellow, and Alexander von Humboldt Fellow.
Dena Dubal is the David A. Coulter Endowed Chair in Ageing and Neurodegenerative Disease at University of California, San Francisco. Dubal has demonstrated that the hormone Klotho can enhance cognition and protect the brain from neurodegenerative decline.
Malú G. Tansey is an American Physiologist and Neuroscientist as well as the Director of the Center for Translational Research in Neurodegenerative Disease at the University of Florida. Tansey holds the titles of Evelyn F. and William L. McKnight Brain Investigator and Norman Fixel Institute for Neurological Diseases Investigator. As the principal investigator of the Tansey Lab, Tansey guides a research program centered around investigating the role of neuroimmune interactions in the development and progression of neurodegenerative and neuropsychiatric disease. Tansey's work is primarily focused on exploring the cellular and molecular basis of peripheral and central inflammation in the pathology of age-related neurodegenerative diseases like Alzheimer's disease and amyotrophic lateral sclerosis.
Sonia Gandhi is a British physician and neuroscientist who leads the Francis Crick Institute neurodegeneration laboratory. She holds a joint position at the UCL Queen Square Institute of Neurology. Her research investigates the molecular mechanisms that give rise to Parkinson's disease. During the COVID-19 pandemic, Gandhi was involved with the epidemiological investigations and testing efforts at the Francis Crick Institute.
Rapid eye movement sleep behaviour disorder and Parkinson's disease is rapid eye movement sleep behavior disorder (RBD) that is associated with Parkinson's disease. RBC is linked genetically and neuropathologically to α- synuclein, a presynaptic neuronal protein that exerts deleterious effects on neighbouring proteins, leading to neuronal death. This pathology is linked to numerous other neurodegenerative disorders, such as Lewy bodies dementia, and collectively these disorders are known as synucleinopathies. Numerous reports over the past few years have stated the frequent association of synucleinopathies with REM sleep behaviour disorder (RBD). In particular, the frequent association of RBD with Parkinson's. In the general population the incidence of RBD is around 0.5%, compared to the prevalence of RBD in PD patients, which has been reported to be between 38% and 60%. The diagnosis and symptom onset of RBD typically precedes the onset of motor or cognitive symptoms of PD by a number of years, typically ranging anywhere from 2 to 15 years prior. Hence, this link could provide an important window of opportunity in the implementation of therapies and treatments, that could prevent or slow the onset of PD.
Glenda Margaret Halliday is an Australian neuroscientist. As of 2021, she is a professor at the University of Sydney and research fellow in the National Health and Medical Research Council (NHMRC). She was named 2022 NSW Scientist of the Year.
Saak Victor Ovsepian is an Armenian-Irish neuroscientist best known for his research in neurobiology, neurotherapeutics and translational biosciences. He is a professor in biosciences at the University of Greenwich.
Lynette Joy Tippett is a New Zealand professor of psychology at the University of Auckland, specialising in neurodegenerative diseases.
References
- 1 2 3 "Changes in neuronal excitability and proteostasis in neurodegenerative disease | News & Events". newsevents.med.unsw.edu.au. Retrieved 2023-06-04.
- ↑ "Professor Lezanne Ooi | Australian Journal of Dementia Care". journalofdementiacare.com. Retrieved 2023-06-04.
- ↑ "2014: Talking brains over a beer - University of Wollongong – UOW". www.uow.edu.au. Retrieved 2023-06-04.
- ↑ "An analysis of repressor element 1- silencing transcription factor interactions with its target genes | WorldCat.org". www.worldcat.org. Retrieved 2023-06-04.
- ↑ "Breakthrough For Treatment Of Fatal Heart Condition". ScienceDaily. Retrieved 2023-06-04.
- ↑ Drewitt-Smith, Ainslie (2020-11-18). "Girl whose rare vanishing white matter disease triggered a search for a cure dies". ABC News. Retrieved 2023-06-04.
- ↑ Latifi, Agron (2020-04-24). "Illawarra walkers rally for Vanishing White Matter Disease research". Illawarra Mercury. Retrieved 2023-06-04.
- ↑ Wachsmuth, Lisa (2020-02-06). "Hope for families after breakthrough into Vanishing White Matter disease". Illawarra Mercury. Retrieved 2023-06-04.
- ↑ Mannix, Liam (2022-10-01). "Does contracting COVID make you more likely to develop dementia?". The Sydney Morning Herald. Retrieved 2023-06-04.
- ↑ "Michael J. Fox and Shake It Up fund scientist's research". Mirage News. 2023-04-03. Retrieved 2023-06-04.
- 1 2 "2022: Cracking the code for Parkinson's, one cell at a time - University of Wollongong – UOW". www.uow.edu.au. Retrieved 2023-06-04.