Insulin resistance (IR) is a pathological condition in which cells either fail to respond normally to the hormone insulin or downregulate insulin receptors in response to hyperinsulinemia.

Lipolysis is the metabolic pathway through which lipid triglycerides are hydrolyzed into a glycerol and free fatty acids. It is used to mobilize stored energy during fasting or exercise, and usually occurs in fat adipocytes. The most important regulatory hormone in lipolysis is insulin; lipolysis can only occur when insulin action falls to low levels, as occurs during fasting. Other hormones that affect lipolysis include leptin, glucagon, epinephrine, norepinephrine, growth hormone, atrial natriuretic peptide, brain natriuretic peptide, and cortisol.
Carbohydrate metabolism is the whole of the biochemical processes responsible for the metabolic formation, breakdown, and interconversion of carbohydrates in living organisms.

Adipose tissue is a loose connective tissue composed mostly of adipocytes. It also contains the stromal vascular fraction (SVF) of cells including preadipocytes, fibroblasts, vascular endothelial cells and a variety of immune cells such as adipose tissue macrophages. Its main role is to store energy in the form of lipids, although it also cushions and insulates the body.

Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Adipocytes are derived from mesenchymal stem cells which give rise to adipocytes through adipogenesis. In cell culture, adipocyte progenitors can also form osteoblasts, myocytes and other cell types.
Adiponectin is a protein hormone and adipokine, which is involved in regulating glucose levels and fatty acid breakdown. In humans, it is encoded by the ADIPOQ gene and is produced primarily in adipose tissue, but also in muscle and even in the brain.

Ceramides are a family of waxy lipid molecules. A ceramide is composed of sphingosine and a fatty acid joined by an amide bond. Ceramides are found in high concentrations within the cell membrane of eukaryotic cells, since they are component lipids that make up sphingomyelin, one of the major lipids in the lipid bilayer. Contrary to previous assumptions that ceramides and other sphingolipids found in cell membrane were purely supporting structural elements, ceramide can participate in a variety of cellular signaling: examples include regulating differentiation, proliferation, and programmed cell death (PCD) of cells.
In biochemistry, lipogenesis is the conversion of fatty acids and glycerol into fats, or a metabolic process through which acetyl-CoA is converted to triglyceride for storage in fat. Lipogenesis encompasses both fatty acid and triglyceride synthesis, with the latter being the process by which fatty acids are esterified to glycerol before being packaged into very-low-density lipoprotein (VLDL). Fatty acids are produced in the cytoplasm of cells by repeatedly adding two-carbon units to acetyl-CoA. Triacylglycerol synthesis, on the other hand, occurs in the endoplasmic reticulum membrane of cells by bonding three fatty acid molecules to a glycerol molecule. Both processes take place mainly in liver and adipose tissue. Nevertheless, it also occurs to some extent in other tissues such as the gut and kidney. A review on lipogenesis in the brain was published in 2008 by Lopez and Vidal-Puig. After being packaged into VLDL in the liver, the resulting lipoprotein is then secreted directly into the blood for delivery to peripheral tissues.
Glucose transporter type 4 (GLUT4), also known as solute carrier family 2, facilitated glucose transporter member 4, is a protein encoded, in humans, by the SLC2A4 gene. GLUT4 is the insulin-regulated glucose transporter found primarily in adipose tissues and striated muscle. The first evidence for this distinct glucose transport protein was provided by David James in 1988. The gene that encodes GLUT4 was cloned and mapped in 1989.
Starvation response in animals is a set of adaptive biochemical and physiological changes, triggered by lack of food or extreme weight loss, in which the body seeks to conserve energy by reducing metabolic rate and/or non-resting energy expenditure to prolong survival and preserve body fat and lean mass.
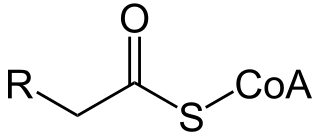
Acyl-CoA is a group of coenzymes that metabolize carboxylic acids. Fatty acyl-CoA's are susceptible to beta oxidation, forming, ultimately, acetyl-CoA. The acetyl-CoA enters the citric acid cycle, eventually forming several equivalents of ATP. In this way, fats are converted to ATP, the common biochemical energy carrier.

White adipose tissue or white fat is one of the two types of adipose tissue found in mammals. The other kind is brown adipose tissue. White adipose tissue is composed of monolocular adipocytes.

The fatty-acid-binding proteins (FABPs) are a family of transport proteins for fatty acids and other lipophilic substances such as eicosanoids and retinoids. These proteins are thought to facilitate the transfer of fatty acids between extra- and intracellular membranes. Some family members are also believed to transport lipophilic molecules from outer cell membrane to certain intracellular receptors such as PPAR. The FABPs are intracellular carriers that “solubilize” the endocannabinoid anandamide (AEA), transporting AEA to the breakdown by FAAH, and compounds that bind to FABPs block AEA breakdown, raising its level. The cannabinoids are also discovered to bind human FABPs that function as intracellular carriers, as THC and CBD inhibit the cellular uptake and catabolism of AEA by targeting FABPs. Competition for FABPs may in part or wholly explain the increased circulating levels of endocannabinoids reported after consumption of cannabinoids. Levels of fatty-acid-binding protein have been shown to decline with ageing in the mouse brain, possibly contributing to age-associated decline in synaptic activity.

Peroxisome proliferator-activated receptor gamma, also known as the glitazone reverse insulin resistance receptor, or NR1C3 is a type II nuclear receptor functioning as a transcription factor that in humans is encoded by the PPARG gene.

Stearoyl-CoA desaturase is an endoplasmic reticulum enzyme that catalyzes the rate-limiting step in the formation of monounsaturated fatty acids (MUFAs), specifically oleate and palmitoleate from stearoyl-CoA and palmitoyl-CoA. Oleate and palmitoleate are major components of membrane phospholipids, cholesterol esters and alkyl-diacylglycerol. In humans, the enzyme is present in two isoforms, encoded respectively by the SCD1 and SCD5 genes.
Fructolysis refers to the metabolism of fructose from dietary sources. Though the metabolism of glucose through glycolysis uses many of the same enzymes and intermediate structures as those in fructolysis, the two sugars have very different metabolic fates in human metabolism. Under one percent of ingested fructose is directly converted to plasma triglyceride. 29% - 54% of fructose is converted in liver to glucose, and about a quarter of fructose is converted to lactate. 15% - 18% is converted to glycogen. Glucose and lactate are then used normally as energy to fuel cells all over the body.

Neutral lipid storage disease is a congenital autosomal recessive disorder characterized by accumulation of triglycerides in the cytoplasm of leukocytes, muscle, liver, fibroblasts, and other tissues. It commonly occurs as one of two subtypes, cardiomyopathic neutral lipid storage disease (NLSD-M), or ichthyotic neutral lipid storage disease (NLSD-I) which is also known as Chanarin–Dorfman syndrome), which are characterized primarily by myopathy and ichthyosis, respectively. Normally, the ichthyosis that is present is typically non-bullous congenital ichthyosiform erythroderma which appears as white scaling.
Intramyocellular lipids are fats stored in droplets in muscle cells. They provide an important energy source for working muscle. During exercise, a large amount of circulating free fatty acids are directed into muscle cells for energy; during rest, incoming fatty acids are instead stored in the muscle cell as triglycerides for later burning. However, an increase in muscle insulin resistance, caused by obesity, diabetes mellitus type 2, and metabolic syndrome, will result in an excess accumulation of intramyocellular lipids.

Perilipin 5, also known as Oxpatperilipin 5 or PLIN5, is a protein that belongs to perilipin family. This protein group has been shown to be responsible for lipid droplet's biogenesis, structure and degradation. In particular, Perilipin 5 is a lipid droplet-associated protein whose function is to keep the balance between lipolysis and lipogenesis, as well as maintaining lipid droplet homeostasis. For example, in oxidative tissues, muscular tissues and cardiac tissues, PLIN5 promotes association between lipid droplets and mitochondria.

Antonio Vidal-Puig is a Spanish medical doctor and scientist who works as a Professor of Molecular Nutrition and Metabolism at the University of Cambridge (UK), best known for advancing the concept that pharmacological targeting of brown fat may serve to treat overweight and obesity in affected individuals, as well as for introducing the concept of adipose tissue "expandability" as an important factor in the pathogenesis of insulin resistance in the context of positive energy balance. His published work focuses on areas such as adipose tissue metabolism and lipotoxicity, regulation of insulin secretion, and the pathophysiology of metabolic syndrome, obesity, and type 2 diabetes. In April 2024, he was granted the rank of doctor honoris causa from the King Juan Carlos University, Madrid.