Related Research Articles
Iogen Corporation is a Canadian company based in Ottawa, Ontario, Canada, and was founded by Patrick Foody Sr. in 1975.

Sodium oxalate, or disodium oxalate, is the sodium salt of oxalic acid with the formula Na2C2O4. It is a white, crystalline, odorless solid, that decomposes above 290 °C.
Americium dioxide (AmO2) is a black compound of americium. In the solid state AmO2 adopts the fluorite, CaF2 structure. It is used as a source of alpha particles.

Diphenyl oxalate is a solid whose oxidation products are responsible for the chemiluminescence in a glowstick. This chemical is the double ester of phenol with oxalic acid. Upon reaction with hydrogen peroxide, 1,2-dioxetanedione is formed, along with release of the two phenols. The dioxetanedione then reacts with a dye molecule, decomposing to form carbon dioxide and leaving the dye in an excited state. As the dye relaxes back to its unexcited state, it releases a photon of visible light.

Clean technology, in short cleantech, is any process, product, or service that reduces negative environmental impacts through significant energy efficiency improvements, the sustainable use of resources, or environmental protection activities. Clean technology includes a broad range of technology related to recycling, renewable energy, information technology, green transportation, electric motors, green chemistry, lighting, grey water, and more. Environmental finance is a method by which new clean technology projects can obtain financing through the generation of carbon credits. A project that is developed with concern for climate change mitigation is also known as a carbon project.
Electrosynthesis in chemistry is the synthesis of chemical compounds in an electrochemical cell. Compared to ordinary redox reaction, electrosynthesis sometimes offers improved selectivity and yields. Electrosynthesis is actively studied as a science and also has industrial applications. Electrooxidation has potential for wastewater treatment as well.

Silver oxalate is commonly employed in experimental petrology to add carbon dioxide to experiments as it will break down to silver (Ag) and carbon dioxide under geologic conditions. It is also a precursor to the production of silver nanoparticles. It is explosive upon heating around 140 degrees Celsius, shock or friction.
GreenFuel Technologies Corporation (GFT) was a startup that developed a process of growing algae using emissions from fossil fuels to produce biofuel from algae.
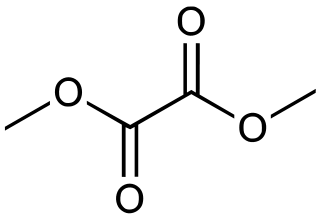
Dimethyl oxalate is the organic compound with the formula (CO2CH3)2. It is the dimethyl ester of oxalic acid. Dimethyl oxalate is a colorless or white solid that is soluble in water.

Dioxane tetraketone (or 1,4-dioxane-2,3,5,6-tetrone) is an organic compound with the formula C4O6. It is an oxide of carbon (an oxocarbon), which can be viewed as the fourfold ketone of dioxane. It can also be viewed as the cyclic dimer of oxiranedione (C2O3), the hypothetical anhydride of oxalic acid.
The electrochemical reduction of carbon dioxide, also known as electrolysis of carbon dioxide, is the conversion of carbon dioxide to more reduced chemical species using electrical energy. It is one possible step in the broad scheme of carbon capture and utilization, nevertheless it is deemed to be one of the most promising approaches.

Dr. George Philippidis is a renewable energy and sustainability leader, who has published and spoken extensively about the global need for renewable energy as the foundation of a green economy and a sustainable society. He advocates the development of renewable power and fuels to enhance energy security, combat climate change, and secure sustainable economic growth. He has authored 11 cleantech patents, written numerous articles, and spoken nationally and internationally emphasizing that renewable energy can initially supplement and augment current resources and progressively replace fossil energy based on its own merits rather than on government policy.

Andrew Bruce Bocarsly is currently a professor at Princeton University, New Jersey. His primary research interests lie in physical inorganic chemistry. He conducts research in electrochemistry, photochemistry, solids state chemistry, and fuel cells, and is known for his work on alternate energy solutions involving processes and materials for photo-reduction and electro-reduction.
Photoelectrochemical reduction of carbon dioxide, also known as photoelectrolysis of carbon dioxide, is a chemical process whereby carbon dioxide is reduced to carbon monoxide or hydrocarbons by the energy of incident light. This process requires catalysts, most of which are semiconducting materials. The feasibility of this chemical reaction was first theorised by Giacomo Luigi Ciamician, an Italian photochemist. Already in 1912 he stated that "[b]y using suitable catalyzers, it should be possible to transform the mixture of water and carbon dioxide into oxygen and methane, or to cause other endo-energetic processes."

Electrofuels, also known as e-fuels or synthetic fuels, are a type of drop-in replacement fuel. They are manufactured using captured carbon dioxide or carbon monoxide, together with hydrogen obtained from sustainable electricity sources such as wind, solar and nuclear power.

Carbon-neutral fuel is fuel which produces no net-greenhouse gas emissions or carbon footprint. In practice, this usually means fuels that are made using carbon dioxide (CO2) as a feedstock. Proposed carbon-neutral fuels can broadly be grouped into synthetic fuels, which are made by chemically hydrogenating carbon dioxide, and biofuels, which are produced using natural CO2-consuming processes like photosynthesis.
E-diesel is a synthetic diesel fuel created by Audi for use in automobiles. Currently, e-diesel is created by an Audi research facility in partnership with a company named Sunfire. The fuel is created from carbon dioxide, water, and electricity with a process powered by renewable energy sources to create a liquid energy carrier called blue crude which is then refined to generate e-diesel. E-diesel is considered to be a carbon-neutral fuel as it does not extract new carbon and the energy sources to drive the process are from carbon-neutral sources. As of April 2015, an Audi A8 driven by Federal Minister of Education and Research in Germany is using the e-diesel fuel.

Florence Gschwend is a Swiss chemical engineer and Royal Academy of Engineering Enterprise Fellow at Imperial College London. She is the founder and CEO of Lixea, a spin-out company that commercialises wood fractionation to enable a circular bioeconomy.

Carbon capture and utilization (CCU) is the process of capturing carbon dioxide (CO2) to be recycled for further usage. Carbon capture and utilization may offer a response to the global challenge of significantly reducing greenhouse gas emissions from major stationary (industrial) emitters. CCU differs from carbon capture and storage (CCS) in that CCU does not aim nor result in permanent geological storage of carbon dioxide. Instead, CCU aims to convert the captured carbon dioxide into more valuable substances or products; such as plastics, concrete or biofuel; while retaining the carbon neutrality of the production processes.

Lynden A. Archer is a chemical engineer, Joseph Silbert Dean of Engineering, David Croll Director of the Energy Systems Institute, and professor of chemical engineering at Cornell University. He became a fellow of the American Physical Society in 2007 and was elected into the National Academy of Engineering in 2018. Archer's research covers polymer and hybrid materials and finds applications in energy storage technologies. His h-index is 92 by Google Scholar.
References
- 1 2 3 Lamonica, Martin. "A Cheaper Route to Making Chemicals from CO2". technologyreview.com. MIT. Retrieved 10 January 2015.
- 1 2 Retka Schill, Susanne. "NJ company unveils low-cost CO2-to-chemical process". ethanolproducer.com. BBI International. Retrieved 10 January 2015.
- ↑ Knapp, Alan. "Liquid Light Raises $15 Million To Convert Carbon Emissions To Chemicals". Forbes. Retrieved 10 January 2015.
- ↑ "New Fundings, Accolades Shine on CO2-to-Chemicals Startup Liquid Light". cleantechiq.com. CLEANTECHIQ. Retrieved 10 January 2015.
- ↑ Hodson, Hal. "Don't waste CO2, turn it into bottles and glue". newscientist.com. Reed Business Information Ltd. Retrieved 10 January 2015.
- ↑ Andrei, Mihai. "Seeing CO2 as a resource: using it for superglue, bottles and fertilizer". zmescience.com. ZME Science. Retrieved 10 January 2015.
- 1 2 Analyst, CTIQ. "New Fundings, Accolades Shine on CO2-to-Chemicals Startup Liquid Light". cleantechiq.com. CleanTechIQ. Retrieved 15 November 2014.
- ↑ Zandonella, Catherine. "Startup born in Princeton lab turns carbon dioxide into fuels". princeton.edu. News at Princeton. Retrieved 15 November 2014.
- ↑ Lane, Jim. "Liquid Light, Virent, Rivertop Renewables, NexSteppe take top slots in The 40 Hottest Smaller Companies in the Advanced Bioeconomy for 2014-15". biofuelsdigest.com. Biofuels Digest. Retrieved 15 November 2014.
- ↑ "Climate Change and Emissions Management Corporation (CCEMC) names winners for round one of $35 million international Carbon Use competition". ccemc.ca. CCEMC. Retrieved 15 November 2014.
- ↑ "Global Cleantech 100 Awards". cleantech.com. Cleantech Group. Retrieved 15 November 2014.
- 1 2 Lane, Jim. "Liquid Light raises $15M and a lot of eyebrows as it advances toward making $$ out of waste CO2". biofuelsdigest.com. Biofuels Digest. Retrieved 15 November 2014.
- ↑ "Avantium Acquires Liquid Light". avantium.com. Retrieved 11 August 2022.