Related Research Articles

Ribosomes are macromolecular machines, found within all cells, that perform biological protein synthesis. Ribosomes link amino acids together in the order specified by the codons of messenger RNA molecules to form polypeptide chains. Ribosomes consist of two major components: the small and large ribosomal subunits. Each subunit consists of one or more ribosomal RNA molecules and many ribosomal proteins. The ribosomes and associated molecules are also known as the translational apparatus.

In biology, translation is the process in living cells in which proteins are produced using RNA molecules as templates. The generated protein is a sequence of amino acids. This sequence is determined by the sequence of nucleotides in the RNA. The nucleotides are considered three at a time. Each such triple results in addition of one specific amino acid to the protein being generated. The matching from nucleotide triple to amino acid is called the genetic code. The translation is performed by a large complex of functional RNA and proteins called ribosomes. The entire process is called gene expression.
The 5′ untranslated region is the region of a messenger RNA (mRNA) that is directly upstream from the initiation codon. This region is important for the regulation of translation of a transcript by differing mechanisms in viruses, prokaryotes and eukaryotes. While called untranslated, the 5′ UTR or a portion of it is sometimes translated into a protein product. This product can then regulate the translation of the main coding sequence of the mRNA. In many organisms, however, the 5′ UTR is completely untranslated, instead forming a complex secondary structure to regulate translation.
The Shine–Dalgarno (SD) sequence is a ribosomal binding site in bacterial and archaeal messenger RNA, generally located around 8 bases upstream of the start codon AUG. The RNA sequence helps recruit the ribosome to the messenger RNA (mRNA) to initiate protein synthesis by aligning the ribosome with the start codon. Once recruited, tRNA may add amino acids in sequence as dictated by the codons, moving downstream from the translational start site.

John Shine is an Australian biochemist and molecular biologist. Shine and Lynn Dalgarno discovered a nucleotide sequence, called the Shine-Dalgarno sequence, necessary for the initiation of protein synthesis. He directed the Garvan Institute of Medical Research in Sydney from 1990 to 2011. From 2018 to 2022, Shine was President of the Australian Academy of Science.

Ribosomal ribonucleic acid (rRNA) is a type of non-coding RNA which is the primary component of ribosomes, essential to all cells. rRNA is a ribozyme which carries out protein synthesis in ribosomes. Ribosomal RNA is transcribed from ribosomal DNA (rDNA) and then bound to ribosomal proteins to form small and large ribosome subunits. rRNA is the physical and mechanical factor of the ribosome that forces transfer RNA (tRNA) and messenger RNA (mRNA) to process and translate the latter into proteins. Ribosomal RNA is the predominant form of RNA found in most cells; it makes up about 80% of cellular RNA despite never being translated into proteins itself. Ribosomes are composed of approximately 60% rRNA and 40% ribosomal proteins, though this ratio differs between prokaryotes and eukaryotes.
Christopher John Leaver is an Emeritus Professorial Fellow of St John's College, Oxford who served as Sibthorpian Professor in the Department of Plant Sciences at the University of Oxford from 1990 to 2007.
An internal ribosome entry site, abbreviated IRES, is an RNA element that allows for translation initiation in a cap-independent manner, as part of the greater process of protein synthesis. Initiation of eukaryotic translation nearly always occurs at and is dependent on the 5' cap of mRNA molecules, where the translation initiation complex forms and ribosomes engage the mRNA. IRES elements, however allow ribosomes to engage the mRNA and begin translation independently of the 5' cap.
Bacterial translation is the process by which messenger RNA is translated into proteins in bacteria.
The Kozak consensus sequence is a nucleic acid motif that functions as the protein translation initiation site in most eukaryotic mRNA transcripts. Regarded as the optimum sequence for initiating translation in eukaryotes, the sequence is an integral aspect of protein regulation and overall cellular health as well as having implications in human disease. It ensures that a protein is correctly translated from the genetic message, mediating ribosome assembly and translation initiation. A wrong start site can result in non-functional proteins. As it has become more studied, expansions of the nucleotide sequence, bases of importance, and notable exceptions have arisen. The sequence was named after the scientist who discovered it, Marilyn Kozak. Kozak discovered the sequence through a detailed analysis of DNA genomic sequences.

In molecular biology, the 5.8S ribosomal RNA is a non-coding RNA component of the large subunit of the eukaryotic ribosome and so plays an important role in protein translation. It is transcribed by RNA polymerase I as part of the 45S precursor that also contains 18S and 28S rRNA. Its function is thought to be in ribosome translocation. It is also known to form covalent linkage to the p53 tumour suppressor protein. 5.8S rRNA can be used as a reference gene for miRNA detection. The 5.8S ribosomal RNA is used to better understand other rRNA processes and pathways in the cell.
A ribosome binding site, or ribosomal binding site (RBS), is a sequence of nucleotides upstream of the start codon of an mRNA transcript that is responsible for the recruitment of a ribosome during the initiation of translation. Mostly, RBS refers to bacterial sequences, although internal ribosome entry sites (IRES) have been described in mRNAs of eukaryotic cells or viruses that infect eukaryotes. Ribosome recruitment in eukaryotes is generally mediated by the 5' cap present on eukaryotic mRNAs.

The prokaryotic small ribosomal subunit, or 30S subunit, is the smaller subunit of the 70S ribosome found in prokaryotes. It is a complex of the 16S ribosomal RNA (rRNA) and 19 proteins. This complex is implicated in the binding of transfer RNA to messenger RNA (mRNA). The small subunit is responsible for the binding and the reading of the mRNA during translation. The small subunit, both the rRNA and its proteins, complexes with the large 50S subunit to form the 70S prokaryotic ribosome in prokaryotic cells. This 70S ribosome is then used to translate mRNA into proteins.
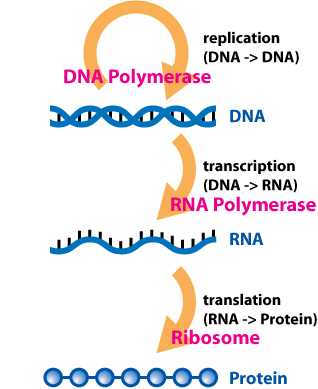
In molecular genetics, an untranslated region refers to either of two sections, one on each side of a coding sequence on a strand of mRNA. If it is found on the 5' side, it is called the 5' UTR, or if it is found on the 3' side, it is called the 3' UTR. mRNA is RNA that carries information from DNA to the ribosome, the site of protein synthesis (translation) within a cell. The mRNA is initially transcribed from the corresponding DNA sequence and then translated into protein. However, several regions of the mRNA are usually not translated into protein, including the 5' and 3' UTRs.

60S ribosomal protein L41 is a protein that is specific to humans and is encoded by the RPL41 gene, also known as HG12 and large eukaryotic ribosomal subunit protein eL41. The gene family HGNC is L ribosomal proteins. The protein itself is also described as P62945-RL41_HUMAN on the GeneCards database. This RPL41 gene is located on chromosome 12.

39S ribosomal protein L12, mitochondrial is a protein that in humans is encoded by the MRPL12 gene.
Translational regulation refers to the control of the levels of protein synthesized from its mRNA. This regulation is vastly important to the cellular response to stressors, growth cues, and differentiation. In comparison to transcriptional regulation, it results in much more immediate cellular adjustment through direct regulation of protein concentration. The corresponding mechanisms are primarily targeted on the control of ribosome recruitment on the initiation codon, but can also involve modulation of peptide elongation, termination of protein synthesis, or ribosome biogenesis. While these general concepts are widely conserved, some of the finer details in this sort of regulation have been proven to differ between prokaryotic and eukaryotic organisms.
Numerous key discoveries in biology have emerged from studies of RNA, including seminal work in the fields of biochemistry, genetics, microbiology, molecular biology, molecular evolution, and structural biology. As of 2010, 30 scientists have been awarded Nobel Prizes for experimental work that includes studies of RNA. Specific discoveries of high biological significance are discussed in this article.

Ribosomal pause refers to the queueing or stacking of ribosomes during translation of the nucleotide sequence of mRNA transcripts. These transcripts are decoded and converted into an amino acid sequence during protein synthesis by ribosomes. Due to the pause sites of some mRNA's, there is a disturbance caused in translation. Ribosomal pausing occurs in both eukaryotes and prokaryotes. A more severe pause is known as a ribosomal stall.
Archaeal translation is the process by which messenger RNA is translated into proteins in archaea. Not much is known on this subject, but on the protein level it seems to resemble eukaryotic translation.
References
- ↑ "Family Notices, Births: Dalgarno". Argus. 16 November 1935. Retrieved 6 June 2023.
- ↑ "Four Year Old Traveller on Flying Boat". Telegraph. 14 March 1940. p. 6. Retrieved 6 June 2023.
- ↑ Dalgarno, L.; Hird, F. J. R. (1 August 1960). "Increase in the process of accumulation of amino acids in carrot slices with prolonged aerobic washing". Biochemical Journal. 76 (2): 209–215. doi:10.1042/bj0760209. ISSN 0306-3283. PMC 1204694 . PMID 13813809.
- ↑ Dalgarno, Lynn (1962). Respiratory metabolism and processes of uptake in a plant tissue (Thesis). OCLC 221785353.
- 1 2 3 Flesch, Juliet (1 April 2015). Transforming Biology: A History of the Department of Biochemistry and Molecular Biology at the University of Melbourne. Melbourne: Melbourne University Publishing. pp. 130–133. ISBN 978-0-522-86771-8.
- ↑ "CNRS / Institut de Biologie Physico-Chimique". istc.int. Archived from the original on 27 March 2020. Retrieved 4 June 2022.
- ↑ Bygrave, Fye (2000). "Personal Recollections of Fyfe Bygrave, The Department of Biochemistry 1967-2000 - Now the Division of Biochemistry And Molecular Biology, Faculty of Science, the Australian National University" (PDF). anu.edu.au. Archived (PDF) from the original on 23 March 2022. Retrieved 4 June 2022.
- ↑ Ja, Crystal (17 November 2010). "'Father of cloning' gets $300,000 prize". The Sydney Morning Herald. Retrieved 4 June 2022.
- ↑ Holm, Carl (17 November 2010). "Genetech pioneer awarded science prize". www.abc.net.au. Retrieved 4 June 2022.
- 1 2 3 "The Shine-Dalgarno sequence - the beginnings of biotechnology | ANU Research School of Biology". biology.anu.edu.au. Retrieved 4 June 2022.