Related Research Articles
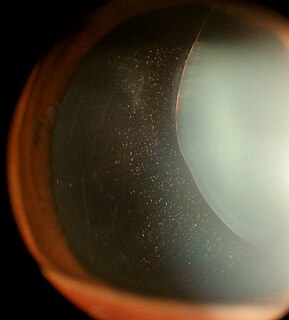
Marfan syndrome (MFS) is a multi-systemic genetic disorder that affects the connective tissue. Those with the condition tend to be tall and thin, with long arms, legs, fingers, and toes. They also typically have overly-flexible joints and scoliosis. The most serious complications involve the heart and aorta, with an increased risk of mitral valve prolapse and aortic aneurysm. The lungs, eyes, bones, and the covering of the spinal cord are also commonly affected. The severity of the symptoms is variable.

Elastin is a protein that in humans is encoded by the ELN gene. Elastin is a key component of the extracellular matrix in gnathostomes. It is highly elastic and present in connective tissue allowing many tissues in the body to resume their shape after stretching or contracting. Elastin helps skin to return to its original position when it is poked or pinched. Elastin is also an important load-bearing tissue in the bodies of vertebrates and used in places where mechanical energy is required to be stored.

An aortic aneurysm is an enlargement (dilatation) of the aorta to greater than 1.5 times normal size. They usually cause no symptoms except when ruptured. Occasionally, there may be abdominal, back, or leg pain. The prevalence of abdominal aortic aneurysm ("AAA") has been reported to range from 2 to 12% and is found in about 8% of men more than 65 years of age. The mortality rate attributable to AAA is about 15,000 per year in the United States and 6,000 to 8,000 per year in the United Kingdom and Ireland. Between 2001 and 2006, there were approximately 230,000 AAA surgical repairs performed on Medicare patients in the United States.

A thoracic aortic aneurysm is an aortic aneurysm that presents primarily in the thorax.
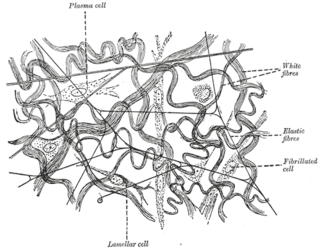
Elastic fibers are an essential component of the extracellular matrix composed of bundles of proteins (elastin) which are produced by a number of different cell types including fibroblasts, endothelial, smooth muscle, and airway epithelial cells. These fibers are able to stretch many times their length, and snap back to their original length when relaxed without loss of energy. Elastic fibers include elastin, elaunin and oxytalan.

Fibrillin is a glycoprotein, which is essential for the formation of elastic fibers found in connective tissue. Fibrillin is secreted into the extracellular matrix by fibroblasts and becomes incorporated into the insoluble microfibrils, which appear to provide a scaffold for deposition of elastin.
A microfibril is a very fine fibril, or fiber-like strand, consisting of glycoproteins and cellulose. It is usually, but not always, used as a general term in describing the structure of protein fiber, e.g. hair and sperm tail. Its most frequently observed structural pattern is the 9+2 pattern in which two central protofibrils are surrounded by nine other pairs. Cellulose inside plants is one of the examples of non-protein compounds that are using this term with the same purpose. Cellulose microfibrils are laid down in the inner surface of the primary cell wall. As the cell absorbs water, its volume increases and the existing microfibrils separate and new ones are formed to help increase cell strength.
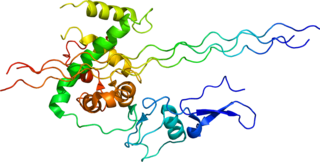
Type III Collagen is a homotrimer, or a protein composed of three identical peptide chains (monomers), each called an alpha 1 chain of type III collagen. Formally, the monomers are called collagen type III, alpha-1 chain and in humans are encoded by the COL3A1 gene. Type III collagen is one of the fibrillar collagens whose proteins have a long, inflexible, triple-helical domain.

MASS syndrome is a medical disorder of the connective tissue similar to Marfan syndrome. MASS stands for: Mitral valve prolapse, Aortic root diameter at upper limits of normal for body size, Stretch marks of the skin, and Skeletal conditions similar to Marfan syndrome. It is caused by a mutation in the FBN1 gene, which encodes fibrillin-1. Fibrillin-1 is an extracellular matrix protein that is found in microfibrils; defects in the fibrillin-1 protein cause the malfunctioning of microfibrils, which results in improper stretching of ligaments, blood vessels, and skin.

Fibrillin-1 is a protein that in humans is encoded by the FBN1 gene, located on chromosome 15. It is a large, extracellular matrix glycoprotein that serves as a structural component of 10-12 nm calcium-binding microfibrils. These microfibrils provide force bearing structural support in elastic and nonelastic connective tissue throughout the body. Mutations altering the protein can result in a variety of phenotypic effects differing widely in their severity, including fetal death, developmental problems, Marfan syndrome or in some cases Weill-Marchesani syndrome.

Collagenase 3 is an enzyme that in humans is encoded by the MMP13 gene. It is a member of the matrix metalloproteinase (MMP) family. Like most MMPs, it is secreted as an inactive pro-form. MMP-13 has an predicted molecular weight around 54 kDa. It is activated once the pro-domain is cleaved, leaving an active enzyme composed of the catalytic domain and the hemopexin-like domain PDB: 1PEX. Although the actual mechanism has not been described, the hemopexin domain participates in collagen degradation, the catalytic domain alone being particularly inefficient in collagen degradation. During embryonic development, MMP-13 is expressed in the skeleton as required for restructuring the collagen matrix for bone mineralization. In pathological situations it is highly overexpressed; this occurs in human carcinomas, rheumatoid arthritis and osteoarthritis.

Fibulin-5 is a protein that in humans is encoded by the FBLN5 gene.

Fibulin-2 is a protein that in humans is encoded by the FBLN2 gene.
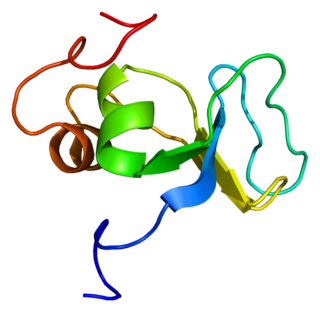
Latent-transforming growth factor beta-binding protein 1 is a protein that in humans is encoded by the LTBP1 gene.

Microfibrillar-associated protein 2 is a protein that in humans is encoded by the MFAP2 gene.

Elastin microfibril interfacer 1 (EMILIN-1) is a protein that in humans is encoded by the EMILIN1 gene. It is the best characterized member of the EMILIN family of extracellular matrix glycoproteins.

Extracellular matrix protein FRAS1 is a protein that in humans is encoded by the FRAS1 gene. This gene encodes an extracellular matrix protein that appears to function in the regulation of epidermal-basement membrane adhesion and organogenesis during development.

Microfibrillar-associated protein 5 is a protein that in humans is encoded by the MFAP5 gene.
Wrinkly skin syndrome(WSS) is a rare genetic condition characterized by sagging, wrinkled skin, low skin elasticity, and delayed fontanel closure along with a range of other symptoms. The disorder exhibits an autosomal recessive inheritance pattern with mutations in the ATP6V0A2 gene, leading to abnormal glycosylation events. There are only about 30 known cases of WSS as of 2010. Given its rarity and symptom overlap to other dermatological conditions, reaching an accurate diagnosis is difficult and requires specialized dermatological testing. Limited treatment options are available but long-term prognosis is variable from patient-to-patient, on the basis of individual case studies. Some skin symptoms recede with increasing age while progressive neurological advancement of the disorder causes seizures and mental deterioration later in life for some patients.
Asprosin is a protein hormone produced by mammals in tissues that stimulates the liver to release glucose into the blood stream. Asprosin is encoded by the gene FBN1 as part of the protein profibrillin and is released from the C-terminus of the latter by specific proteolysis. In the liver, asprosin activates rapid glucose release via a cyclic adenosine monophosphate (cAMP)-dependent pathway.
References
- ↑ "MFAP4 - Microfibril-associated glycoprotein 4 precursor - Homo sapiens (Human) - MFAP4 gene & protein".
- ↑ Pilecki B, et al. (Jan 2016). "Characterization of microfibrillar-associated protein 4 (MFAP4) as a tropoelastin- and fibrillin-binding protein involved in elastic fiber formation". Journal of Biological Chemistry. 291 (3): 1103–14. doi: 10.1074/jbc.M115.681775 . PMC 4714194 . PMID 26601954.
- ↑ "Tissue expression of MFAP4 - Summary - The Human Protein Atlas". www.proteinatlas.org.
- ↑ Schlosser, et al. (Jan 2016). "MFAP4 Promotes Vascular Smooth Muscle Migration, Proliferation and Accelerates Neointima Formation". Arteriosclerosis, Thrombosis and Vascular Biology. 36 (1): 122–33. doi: 10.1161/ATVBAHA.115.306672 . PMID 26564819. S2CID 1740441.
- ↑ Pilecki, et al. (Sep 2015). "Microfibrillar-associated Protein 4 Modulates Airway Smooth Muscle Cell Phenotype in Experimental Asthma". Thorax. 70 (9): 862–72. doi: 10.1136/thoraxjnl-2014-206609 . PMID 26038533. S2CID 207032473.
- 1 2 Yin, et al. (Sep 2019). "Glycoproteomic Analysis of the Aortic Extracellular Matrix in Marfan Patients". Arteriosclerosis, Thrombosis and Vascular Biology. 39 (9): 1859–1873. doi:10.1161/ATVBAHA.118.312175. PMC 6727943 . PMID 31315432.
- ↑ Lindholt, et al. (Jun 2020). "High Plasma Microfibrillar-Associated Protein 4 Is Associated With Reduced Surgical Repair in Abdominal Aortic Aneurysms". Journal of Vascular Surgery. 71 (6): 1921–1929. doi: 10.1016/j.jvs.2019.08.253 . PMID 31784280.
- ↑ Wulf-Johansson, et al. (Dec 2013). "Localization of Microfibrillar-Associated Protein 4 (MFAP4) in Human Tissues: Clinical Evaluation of Serum MFAP4 and Its Association With Various Cardiovascular Conditions". PLOS ONE. 8 (12): e82243. Bibcode:2013PLoSO...882243W. doi: 10.1371/journal.pone.0082243 . PMC 3862580 . PMID 24349233.
- ↑ Johansson, et al. (Sep 2014). "Microfibrillar-associated Protein 4: A Potential Biomarker of Chronic Obstructive Pulmonary Disease". Respiratory Medicine. 108 (9): 1336–44. doi: 10.1016/j.rmed.2014.06.003 . PMID 25022422.
- ↑ Saekmose, et al. (Oct 2015). "Microfibrillar-Associated Protein 4: A Potential Biomarker for Screening for Liver Fibrosis in a Mixed Patient Cohort". PLOS ONE. 10 (10): e0140418. Bibcode:2015PLoSO..1040418S. doi: 10.1371/journal.pone.0140418 . PMC 4604125 . PMID 26460565.
- ↑ Yang, et al. (Jan 2019). "Integrated Analysis of Microfibrillar-Associated Proteins Reveals MFAP4 as a Novel Biomarker in Human Cancers". Epigenomics. 11 (1): 1635–1651. doi: 10.2217/epi-2018-0080 . PMID 30089404.