
An antacid is a substance which neutralizes stomach acidity and is used to relieve heartburn, indigestion, or an upset stomach. Some antacids have been used in the treatment of constipation and diarrhea. Marketed antacids contain salts of aluminum, calcium, magnesium, or sodium. Some preparations contain a combination of two salts, such as magnesium carbonate and aluminum hydroxide.
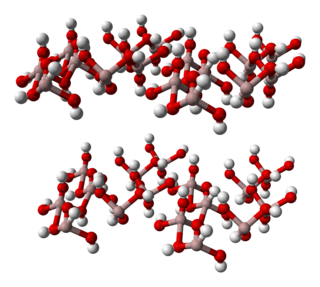
Aluminium hydroxide, Al(OH)3, is found in nature as the mineral gibbsite and its three much rarer polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is amphoteric, i.e., it has both basic and acidic properties. Closely related are aluminium oxide hydroxide, AlO(OH), and aluminium oxide or alumina, the latter of which is also amphoteric. These compounds together are the major components of the aluminium ore bauxite. Aluminium hydroxide also forms a gelatinous precipitate in water.

Magnesium hydroxide is an inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (Ksp = 5.61×10−12). Magnesium hydroxide is a common component of antacids, such as milk of magnesia.
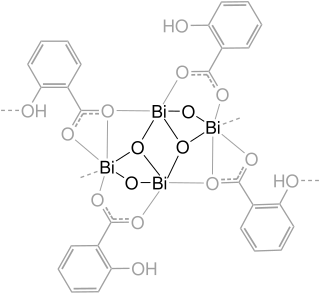
Bismuth subsalicylate, sold generically as pink bismuth and under brand names including Pepto-Bismol, Pepti-Calm and BisBacter, is a medication used to treat temporary discomfort of the stomach and gastrointestinal tract. This includes an upset stomach, heartburn or other similar symptoms.
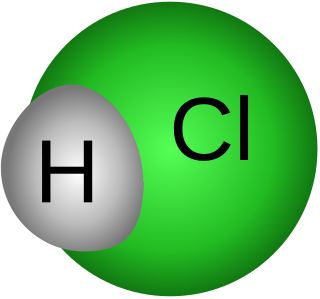
Achlorhydria and hypochlorhydria refer to states where the production of hydrochloric acid in gastric secretions of the stomach and other digestive organs is absent or low, respectively. It is associated with various other medical problems.
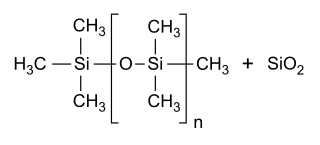
Simeticone (INN), also known as simethicone (USAN), is an anti-foaming agent used to reduce bloating, discomfort or pain caused by excessive gas.
A gastrointestinal cocktail,, is a mixture of medications used to treat symptoms of dyspepsia. The GI cocktail generally contains a mixture of viscous lidocaine, an antacid, and an anticholinergic. The GI cocktail is commonly prescribed in the hospital or emergency department, and has been used to help distinguish chest pain as either gastrointestinal or cardiac. While it has been widely used in the treatment of dyspepsia, studies have suggested that the GI cocktail is only as effective as antacids alone.
Mylanta is a brand of over-the-counter drugs for digestive problems, manufactured by Infirst Healthcare USA under license from McNeil Consumer Healthcare, a division of Kenvue. It includes several different products intended to treat heartburn and bloating.

Rolaids is an American brand of calcium and magnesium-based antacid produced by Procter & Gamble. It was invented by American chemist Irvine W. Grote in the late 1920s, and originated with manufacturing in Chattanooga, Tennessee, under one of Chattem's forerunner companies, which manufactured the brand for Warner-Lambert; Warner-Lambert merged with Pfizer in 2000.
Abdominal bloating is a short-term disease that affects the gastrointestinal tract. Bloating is generally characterized by an excess buildup of gas, air or fluids in the stomach. A person may have feelings of tightness, pressure or fullness in the stomach; it may or may not be accompanied by a visibly distended abdomen. Bloating can affect anyone of any age range and is usually self-diagnosed, in most cases does not require serious medical attention or treatment. Although this term is usually used interchangeably with abdominal distension, these symptoms probably have different pathophysiological processes, which are not fully understood.

Abdominal distension occurs when substances, such as air (gas) or fluid, accumulate in the abdomen causing its expansion. It is typically a symptom of an underlying disease or dysfunction in the body, rather than an illness in its own right. People with this condition often describe it as "feeling bloated". Affected people often experience a sensation of fullness, abdominal pressure, and sometimes nausea, pain, or cramping. In the most extreme cases, upward pressure on the diaphragm and lungs can also cause shortness of breath. Through a variety of causes, bloating is most commonly due to buildup of gas in the stomach, small intestine, or colon. The pressure sensation is often relieved, or at least lessened, by belching or flatulence. Medications that settle gas in the stomach and intestines are also commonly used to treat the discomfort and lessen the abdominal distension.
Loperamide/simethicone is combination medication sold under the brand name Imodium Multi-Symptom Relief used to treat diarrhea and gas simultaneously. It is manufactured by the McNeil Consumer Healthcare Division of McNeil PPC, Inc. It contains loperamide and simethicone.

Lubiprostone, sold under the brand name Amitiza among others, is a medication used in the management of chronic idiopathic constipation, predominantly irritable bowel syndrome-associated constipation in women and opioid-induced constipation. The drug is owned by Mallinckrodt and is marketed by Takeda Pharmaceutical Company.

Polycarbophil calcium (INN) is a drug used as a stool stabilizer. Chemically, it is a synthetic polymer of polyacrylic acid cross-linked with divinyl glycol, with calcium as a counter-ion.
Magnesium salts are available as a medication in a number of formulations. They are used to treat magnesium deficiency, low blood magnesium, eclampsia, and several other conditions. Magnesium is an essential nutrient.
Insulin degludec/liraglutide, sold under the brand name Xultophy, is a fixed-dose combination medication for the treatment of adults with type 2 diabetes to improve glycemic control in combination with diet and exercise. It contains insulin degludec and liraglutide. It is administered by subcutaneous injection.
Healthy digestion, also called digestive health, results in the absorption of nutrients from food without distressing symptoms. Healthy digestion follows having a healthy diet, doing appropriate self-care including physical activity and exercise, minimizing activities like smoking or consuming alcoholic drinks which impair digestion, and managing any medical condition which disrupts digestion to the best of one's ability.
William H. Rorer, Inc was an American multinational pharmaceutical company headquartered in Fort Washington, Pennsylvania. It was founded in 1910 by William H. Rorer, a Pennsylvania pharmacist. The business operated as a small concern, primarily selling pain relievers manufactured elsewhere. It wasn't until after World War II that the company's fortunes forever changed with the introduction of Maalox which would become the world's best selling antacid.

Acid peptic diseases, such as peptic ulcers, Zollinger-Ellison syndrome, and gastroesophageal reflux disease, are caused by distinct but overlapping pathogenic mechanisms involving acid effects on mucosal defense. Acid reflux damages the esophageal mucosa and may also cause laryngeal tissue injury, leading to the development of pulmonary symptoms.
Fenofibrate/pravastatin, sold under the brand name Pravafenix, is a combination medication used for the treatment of hypercholesterolemia in adults whose low-density lipoprotein (LDL) cholesterol is already being controlled with pravastatin alone but who still need to improve their cholesterol levels and to reduce their levels of triglycerides. It contains fenofibrate and pravastatin. It is taken by mouth.











