
Leopold Gmelin was a German chemist. Gmelin was a professor at the University of Heidelberg. He worked on the red prussiate and created Gmelin's test, and wrote his Handbook of Chemistry, which over successive editions became a standard reference work still in use.
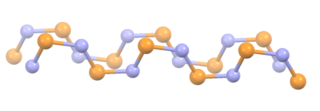
Polythiazyl, (SN)x, is an electrically conductive, gold- or bronze-colored polymer with metallic luster. It was the first conductive inorganic polymer discovered and was also found to be a superconductor at very low temperatures. It is a fibrous solid, described as "lustrous golden on the faces and dark blue-black", depending on the orientation of the sample. It is air stable and insoluble in all solvents.

Chloryl fluoride is the chemical compound with the formula ClO2F. It is commonly encountered as side-product in reactions of chlorine fluorides with oxygen sources. It is the acyl fluoride of chloric acid.
Walter Hieber was an inorganic chemist, known as the father of metal carbonyl chemistry. He was born 18 December 1895 and died 29 November 1976. Hieber's father was Johannes Hieber, an influential evangelical minister and politician.

The Alfred-Stock Memorial Prize or Alfred-Stock-Gedächtnispreis is an award for "an outstanding independent scientific experimental investigation in the field of inorganic chemistry." It is awarded biennially by the German Chemical Society. The award, consisting of a gold medal and money, was created in 1950 in recognition of the pioneering achievements in inorganic chemistry by the German chemist Alfred Stock. In 2022, the GDCh board decided to change the name of the previous Alfred Stock Memorial Prize. The new name is Marianne Baudler Prize.
Wilhelm Karl Klemm was an inorganic and physical chemist. Klemm did extensive work on intermetallic compounds, rare earth metals, transition elements and compounds involving oxygen and fluorine. He and Heinrich Bommer were the first to isolate elemental erbium (1934) and ytterbium (1936). Klemm refined Eduard Zintl's ideas about the structure of intermetallic compounds and their connections to develop the Zintl-Klemm concept.
Marianne Baudler was a German chemist. She is known for her research on phosphorus.
Stefanie Dehnen is a German chemist. She is the executive director of the Institute of Nanotechnology at the Karlsruhe Institute of Technology. From 2006 to 2022, she was a full professor for inorganic chemistry at the University of Marburg. She has received numerous awards for her research in inorganic chemistry. In 2024 and 2025, she will be the president of the German Chemical Society.
Georg Karl Brauer was a German chemist.
Ulrich Müller is a German chemist who is known for his works on solid-state chemistry and the application of crystallographic group theory to crystal chemistry. He is the author of several textbooks on chemistry, solid-state chemistry, and crystallography.

Brigitte Sarry was a German chemist and a professor at Technische Universität Berlin.
Lieselotte Feikes was a German chemist. She is known for her work in the leather chemistry and the development of wastewater treatment processes.
Hans Georg von Schnering was a German chemist and professor of inorganic chemistry at the University of Münster, honorary professor at the University of Stuttgart and director at the Max Planck Institute for Solid State Research.
The inorganic imide is an inorganic chemical compound containing
Nitride fluorides containing nitride and fluoride ions with the formula NF4-. They can be electronically equivalent to a pair of oxide ions O24-. Nitride fluorides were discovered in 1996 by Lavalle et al. They heated diammonium technetium hexafluoride to 300 °C to yield TcNF. Another preparation is to heat a fluoride compound with a nitride compound in a solid state reaction. The fluorimido ion is F-N2- and is found in a rhenium compound.

Cesium sulfide is an inorganic salt with a chemical formula Cs2S. It is a strong alkali in aqueous solution. In the air, cesium sulfide emits rotten egg smelling hydrogen sulfide.
A chloride nitride is a mixed anion compound containing both chloride (Cl−) and nitride ions (N3−). Another name is metallochloronitrides. They are a subclass of halide nitrides or pnictide halides.
An iodide nitride is a mixed anion compound containing both iodide (I−) and nitride ions (N3−). Another name is metalloiodonitrides. They are a subclass of halide nitrides or pnictide halides. Some different kinds include ionic alkali or alkaline earth salts, small clusters where metal atoms surround a nitrogen atom, layered group 4 element 2-dimensional structures, and transition metal nitrido complexes counter-balanced with iodide ions. There is also a family with rare earth elements and nitrogen and sulfur in a cluster.
Carbide iodides are mixed anion compounds containing iodide and carbide anions. Many carbide iodides are cluster compounds, containing one, two or more carbon atoms in a core, surrounded by a layer of metal atoms, and encased in a shell of iodide ions. These ions may be shared between clusters to form chains, double chains or layers.
Walter Rüdorff was a German chemist known for his research on clathrates of graphite and ternary oxides.







