Related Research Articles

The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems.

Nitrification is the biological oxidation of ammonia to nitrate via the intermediary nitrite. Nitrification is an important step in the nitrogen cycle in soil. The process of complete nitrification may occur through separate organisms or entirely within one organism, as in comammox bacteria. The transformation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an aerobic process performed by small groups of autotrophic bacteria and archaea.
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.

Denitrification is a microbially facilitated process where nitrate (NO3−) is reduced and ultimately produces molecular nitrogen (N2) through a series of intermediate gaseous nitrogen oxide products. Facultative anaerobic bacteria perform denitrification as a type of respiration that reduces oxidized forms of nitrogen in response to the oxidation of an electron donor such as organic matter. The preferred nitrogen electron acceptors in order of most to least thermodynamically favorable include nitrate (NO3−), nitrite (NO2−), nitric oxide (NO), nitrous oxide (N2O) finally resulting in the production of dinitrogen (N2) completing the nitrogen cycle. Denitrifying microbes require a very low oxygen concentration of less than 10%, as well as organic C for energy. Since denitrification can remove NO3−, reducing its leaching to groundwater, it can be strategically used to treat sewage or animal residues of high nitrogen content. Denitrification can leak N2O, which is an ozone-depleting substance and a greenhouse gas that can have a considerable influence on global warming.

Microbial ecology is the ecology of microorganisms: their relationship with one another and with their environment. It concerns the three major domains of life—Eukaryota, Archaea, and Bacteria—as well as viruses.

Nitrosomonas is a genus of Gram-negative bacteria, belonging to the Betaproteobacteria. It is one of the five genera of ammonia-oxidizing bacteria and, as an obligate chemolithoautotroph, uses ammonia as an energy source and carbon dioxide as a carbon source in presence of oxygen. Nitrosomonas are important in the global biogeochemical nitrogen cycle, since they increase the bioavailability of nitrogen to plants and in the denitrification, which is important for the release of nitrous oxide, a powerful greenhouse gas. This microbe is photophobic, and usually generate a biofilm matrix, or form clumps with other microbes, to avoid light. Nitrosomonas can be divided into six lineages: the first one includes the species Nitrosomonas europea, Nitrosomonas eutropha, Nitrosomonas halophila, and Nitrosomonas mobilis. The second lineage presents the species Nitrosomonas communis, N. sp. I and N. sp. II, meanwhile the third lineage includes only Nitrosomonas nitrosa. The fourth lineage includes the species Nitrosomonas ureae and Nitrosomonas oligotropha and the fifth and sixth lineages include the species Nitrosomonas marina, N. sp. III, Nitrosomonas estuarii and Nitrosomonas cryotolerans.

Sergei Nikolaevich Winogradsky (Russian: Сергей Николаевич Виноградский; Ukrainian: Сергій Миколайович Виноградський; 13 September [O.S. 1 September] 1856, Kyiv – 24 February 1953, Brie-Comte-Robert), also published under the name Sergius Winogradsky, was a Ukrainian and Russian microbiologist, ecologist and soil scientist who pioneered the cycle-of-life concept. Winogradsky discovered the first known form of lithotrophy during his research with Beggiatoa in 1887. He reported that Beggiatoa oxidized hydrogen sulfide (H2S) as an energy source and formed intracellular sulfur droplets. This research provided the first example of lithotrophy, but not autotrophy. Born in the capital of present-day Ukraine, his legacy is also celebrated by this nation.
Denitrifying bacteria are a diverse group of bacteria that encompass many different phyla. This group of bacteria, together with denitrifying fungi and archaea, is capable of performing denitrification as part of the nitrogen cycle. Denitrification is performed by a variety of denitrifying bacteria that are widely distributed in soils and sediments and that use oxidized nitrogen compounds such as nitrate and nitrite in the absence of oxygen as a terminal electron acceptor. They metabolize nitrogenous compounds using various enzymes, including nitrate reductase (NAR), nitrite reductase (NIR), nitric oxide reductase (NOR) and nitrous oxide reductase (NOS), turning nitrogen oxides back to nitrogen gas or nitrous oxide.
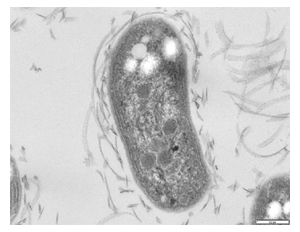
Nitrobacter is a genus comprising rod-shaped, gram-negative, and chemoautotrophic bacteria. The name Nitrobacter derives from the Latin neuter gender noun nitrum, nitri, alkalis; the Ancient Greek noun βακτηρία, βακτηρίᾱς, rod. They are non-motile and reproduce via budding or binary fission. Nitrobacter cells are obligate aerobes and have a doubling time of about 13 hours.

Soil biology is the study of microbial and faunal activity and ecology in soil. Soil life, soil biota, soil fauna, or edaphon is a collective term that encompasses all organisms that spend a significant portion of their life cycle within a soil profile, or at the soil-litter interface. These organisms include earthworms, nematodes, protozoa, fungi, bacteria, different arthropods, as well as some reptiles, and species of burrowing mammals like gophers, moles and prairie dogs. Soil biology plays a vital role in determining many soil characteristics. The decomposition of organic matter by soil organisms has an immense influence on soil fertility, plant growth, soil structure, and carbon storage. As a relatively new science, much remains unknown about soil biology and its effect on soil ecosystems.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.
Paracoccus denitrificans, is a coccoid bacterium known for its nitrate reducing properties, its ability to replicate under conditions of hypergravity and for being a relative of the eukaryotic mitochondrion.

The root microbiome is the dynamic community of microorganisms associated with plant roots. Because they are rich in a variety of carbon compounds, plant roots provide unique environments for a diverse assemblage of soil microorganisms, including bacteria, fungi, and archaea. The microbial communities inside the root and in the rhizosphere are distinct from each other, and from the microbial communities of bulk soil, although there is some overlap in species composition.
Priming or a "priming effect" is said to occur when something that is added to soil or compost affects the rate of decomposition occurring on the soil organic matter (SOM), either positively or negatively. Organic matter is made up mostly of carbon and nitrogen, so adding a substrate containing certain ratios of these nutrients to soil may affect the microbes that are mineralizing SOM. Fertilizers, plant litter, detritus, and carbohydrate exudates from living roots, can potentially positively or negatively prime SOM decomposition.
Nitrogen-15 (15N) tracing is a technique to study the nitrogen cycle using the heavier, stable nitrogen isotope 15N. Despite the different weights, 15N is involved in the same chemical reactions as the more abundant 14N and is therefore used to trace and quantify conversions of one nitrogen compound to another. 15N tracing is applied in biogeochemistry, soil science, environmental science, environmental microbiology and small molecule activation research.

Urine patches in cattle pastures generate large concentrations of the greenhouse gas nitrous oxide through nitrification and denitrification processes in urine-contaminated soils. Over the past few decades, the cattle population has increased more rapidly than the human population. Between the years 2000 and 2050, the cattle population is expected to increase from 1.5 billion to 2.6 billion. When large populations of cattle are packed into pastures, excessive amounts of urine soak into soils. This increases the rate at which nitrification and denitrification occur and produce nitrous oxide. Currently, nitrous oxide is one of the single most important ozone-depleting emissions and is expected to remain the largest throughout the 21st century.
Mary Ann Moran is a distinguished research professor of marine sciences at the University of Georgia in Athens. She studies the role of bacteria in Earth's marine nutrient cycles, and is a leader in the fields of marine sciences and biogeochemistry. Her work is focused on how microbes interact with dissolved organic matter and the impact of microbial diversity on the global carbon and sulfur cycles. By defining the roles of diverse bacteria in the carbon and sulfur cycles, she connects the biogeochemical and organismal approaches in marine science.
Dissimilatory nitrate reduction to ammonium (DNRA), also known as nitrate/nitrite ammonification, is the result of anaerobic respiration by chemoorganoheterotrophic microbes using nitrate (NO3−) as an electron acceptor for respiration. In anaerobic conditions microbes which undertake DNRA oxidise organic matter and use nitrate (rather than oxygen) as an electron acceptor, reducing it to nitrite, and then to ammonium (NO3− → NO2− → NH4+).

The viral shunt is a mechanism that prevents marine microbial particulate organic matter (POM) from migrating up trophic levels by recycling them into dissolved organic matter (DOM), which can be readily taken up by microorganisms. The DOM recycled by the viral shunt pathway is comparable to the amount generated by the other main sources of marine DOM.
Kristen M. DeAngelis is a professor in the department of Microbiology at the University of Massachusetts where she studies soil microbes in relation to climate change.
References
- 1 2 3 4 5 6 "Mary K. Firestone". Dept of Environmental Science, Policy, and Management; University of California, Berkeley. Retrieved 4 May 2018.
- 1 2 Helms, Douglas; Effland, Anne B. W.; Durana, Patricia J. (8 February 2008). Profiles in the History of the U.S. Soil Survey. John Wiley & Sons. ISBN 9780470376737 – via Google Books.
- ↑ "Excellence Honored with CNR Citations". berkeley.edu. Retrieved 4 May 2018.
- ↑ "Mary Firestone". www.nasonline.org. Retrieved 2021-03-20.
- ↑ "Author Details - Firestone, Mary K." SCOPUS. Retrieved 4 May 2018.