Related Research Articles

The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.

Enthalpy is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant external pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work that was done against constant external pressure to establish the system's physical dimensions from to some final volume , i.e. to make room for it by displacing its surroundings. The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; bond, lattice, solvation, and other chemical "energies" are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it.

In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions (cations) and negatively charged ions (anions), which results in a compound with no net electric charge. The constituent ions are held together by electrostatic forces termed ionic bonds.

In thermodynamics, the specific heat capacity of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. It is also referred to as massic heat capacity or as the specific heat. More formally it is the heat capacity of a sample of the substance divided by the mass of the sample. The SI unit of specific heat capacity is joule per kelvin per kilogram, J⋅kg−1⋅K−1. For example, the heat required to raise the temperature of 1 kg of water by 1 K is 4184 joules, so the specific heat capacity of water is 4184 J⋅kg−1⋅K−1.

The melting point of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa.
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state, with all substances in their standard states. The standard pressure value p⦵ = 105 Pa(= 100 kPa = 1 bar) is recommended by IUPAC, although prior to 1982 the value 1.00 atm (101.325 kPa) was used. There is no standard temperature. Its symbol is ΔfH⦵. The superscript Plimsoll on this symbol indicates that the process has occurred under standard conditions at the specified temperature (usually 25 °C or 298.15 K).
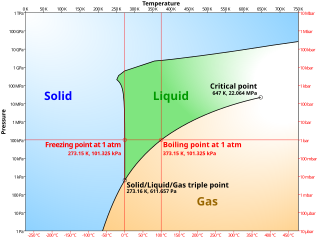
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases occur and coexist at equilibrium.

In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
In chemistry, the standard molar entropy is the entropy content of one mole of pure substance at a standard state of pressure and any temperature of interest. These are often chosen to be the standard temperature and pressure.

In thermodynamics, the Gibbs free energy is a thermodynamic potential that can be used to calculate the maximum amount of work, other than pressure–volume work, that may be performed by a thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as chemical reactions that may occur under these conditions. The Gibbs free energy is expressed as where:
The standard state of a material is a reference point used to calculate its properties under different conditions. A degree sign (°) or a superscript Plimsoll symbol (⦵) is used to designate a thermodynamic quantity in the standard state, such as change in enthalpy (ΔH°), change in entropy (ΔS°), or change in Gibbs free energy (ΔG°). The degree symbol has become widespread, although the Plimsoll is recommended in standards, see discussion about typesetting below.

Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. The verb form of sublimation is sublime, or less preferably, sublimate. Sublimate also refers to the product obtained by sublimation. The point at which sublimation occurs rapidly is called critical sublimation point, or simply sublimation point. Notable examples include sublimation of dry ice at room temperature and atmospheric pressure, and that of solid iodine with heating.

A mill is a device, often a structure, machine or kitchen appliance, that breaks solid materials into smaller pieces by grinding, crushing, or cutting. Such comminution is an important unit operation in many processes. There are many different types of mills and many types of materials processed in them. Historically, mills were powered by hand or by animals, working animal, wind (windmill) or water (watermill). In the modern era, they are usually powered by electricity.
Micronization is the process of reducing the average diameter of a solid material's particles. Traditional techniques for micronization focus on mechanical means, such as milling and grinding. Modern techniques make use of the properties of supercritical fluids and manipulate the principles of solubility.

Cobalt(II) chloride is an inorganic compound, a salt of cobalt and chlorine, with the formula CoCl
2. The compound forms several hydrates CoCl
2·nH
2O, for n = 1, 2, 6, and 9. Claims of the formation of tri- and tetrahydrates have not been confirmed. The anhydrous form is a blue crystalline solid; the dihydrate is purple and the hexahydrate is pink. Commercial samples are usually the hexahydrate, which is one of the most commonly used cobalt salts in the lab.

Deposition is the phase transition in which gas transforms into solid without passing through the liquid phase. Deposition is a thermodynamic process. The reverse of deposition is sublimation and hence sometimes deposition is called desublimation.

The Victor Meyer apparatus is the standard laboratory method for determining the molecular weight of a volatile liquid. It was developed by Viktor Meyer, who spelled his name Victor in publications at the time of its development. In this method, a known mass of a volatile solid or liquid under examination is converted into its vapour form by heating in a Victor Meyer's tube. The vapour displaces its own volume of air. The volume of air displaced at experimental temperature and pressure is calculated. Then volume of air displaced at standard temperature and pressure is calculated. Using this, mass of air displaced at 2.24 × 10−2 m3 of vapour at STP is calculated. This value represents the molecular mass of the substance. The apparatus consists of an inner Victor Meyer's tube, lower end of which is in form of a bulb. The upper end of tube has a side tube that leads to a trough filled with water. The Victor Meyer's tube is surrounded by an outer jacket. In the outer jacket, a liquid is placed, which boils at a temperature at least 30 K higher than the substance under examination. A small quantity of glass-wool or asbestos pad covers the lower end of the Victor Meyer's tube to prevent breakage, when a glass bottle containing the substance under examination is dropped to it

A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a nearly constant volume independent of pressure. It is one of the four fundamental states of matter, and is the only state with a definite volume but no fixed shape.

The S3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. It comprises about 10% of vaporised sulfur at 713 K and 1,333 Pa. It has been observed at cryogenic temperatures as a solid. Under ordinary conditions it converts to cyclooctasulfur.
Polycarbonyl, is a solid, metastable, and explosive polymer of carbon monoxide. The polymer is produced by exposing carbon monoxide to high pressures. The structure of the solid appears amorphous, but may include a zigzag of equally-spaced CO groups.
References
1. Bamfield, Peter and Hutchings, Michael G, Chromic Phenomena: the technological applications of colour chemistry, Royal Society of Chemistry, Cambridge UK, pages 104–5, 2010. ISBN 978-1-84755-868-8.