
An electric current is a flow of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is defined as the net rate of flow of electric charge through a surface. The moving particles are called charge carriers, which may be one of several types of particles, depending on the conductor. In electric circuits the charge carriers are often electrons moving through a wire. In semiconductors they can be electrons or holes. In an electrolyte the charge carriers are ions, while in plasma, an ionized gas, they are ions and electrons.

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

In electromagnetism and electronics, electromotive force is an energy transfer to an electric circuit per unit of electric charge, measured in volts. Devices called electrical transducers provide an emf by converting other forms of energy into electrical energy. Other electrical equipment also produce an emf, such as batteries, which convert chemical energy, and generators, which convert mechanical energy. This energy conversion is achieved by physical forces applying physical work on electric charges. However, electromotive force itself is not a physical force, and ISO/IEC standards have deprecated the term in favor of source voltage or source tension instead.

A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane.
In electrochemistry, the standard hydrogen electrode, is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials. Its absolute electrode potential is estimated to be 4.44 ± 0.02 V at 25 °C, but to form a basis for comparison with all other electrochemical reactions, hydrogen's standard electrode potential is declared to be zero volts at any temperature. Potentials of all other electrodes are compared with that of the standard hydrogen electrode at the same temperature.
In analytical electrochemistry, coulometry determines the amount of matter transformed during an electrolysis reaction by measuring the amount of electricity consumed or produced. It can be used for precision measurements of charge, and the amperes even used to have a coulometric definition. However, today coulometry is mainly used for analytical applications. It is named after Charles-Augustin de Coulomb.
Capillary electrophoresis (CE) is a family of electrokinetic separation methods performed in submillimeter diameter capillaries and in micro- and nanofluidic channels. Very often, CE refers to capillary zone electrophoresis (CZE), but other electrophoretic techniques including capillary gel electrophoresis (CGE), capillary isoelectric focusing (CIEF), capillary isotachophoresis and micellar electrokinetic chromatography (MEKC) belong also to this class of methods. In CE methods, analytes migrate through electrolyte solutions under the influence of an electric field. Analytes can be separated according to ionic mobility and/or partitioning into an alternate phase via non-covalent interactions. Additionally, analytes may be concentrated or "focused" by means of gradients in conductivity and pH.

The copper–copper(II) sulfate electrode is a reference electrode of the first kind, based on the redox reaction with participation of the metal (copper) and its salt, copper(II) sulfate. It is used for measuring electrode potential and is the most commonly used reference electrode for testing cathodic protection corrosion control systems. The corresponding equation can be presented as follow:

The copper coulometer is a one application for the copper-copper(II) sulfate electrode. Such a coulometer consists of two identical copper electrodes immersed in slightly acidic pH-buffered solution of copper(II) sulfate. Passing of current through the element leads to the anodic dissolution of the metal on anode and simultaneous deposition of copper ions on the cathode. These reactions have 100% efficiency over a wide range of current density.
A silver chloride electrode is a type of reference electrode, commonly used in electrochemical measurements. For environmental reasons it has widely replaced the saturated calomel electrode. For example, it is usually the internal reference electrode in pH meters and it is often used as reference in reduction potential measurements. As an example of the latter, the silver chloride electrode is the most commonly used reference electrode for testing cathodic protection corrosion control systems in sea water environments.

Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical data for a voltammetric experiment comes in the form of a voltammogram, which plots the current produced by the analyte versus the potential of the working electrode.
The saturated calomel electrode (SCE) is a reference electrode based on the reaction between elemental mercury and mercury(I) chloride. It has been widely replaced by the silver chloride electrode, however the calomel electrode has a reputation of being more robust. The aqueous phase in contact with the mercury and the mercury(I) chloride (Hg2Cl2, "calomel") is a saturated solution of potassium chloride in water. The electrode is normally linked via a porous frit (sometimes coupled to a salt bridge) to the solution in which the other electrode is immersed.
Polarography is a type of voltammetry where the working electrode is a dropping mercury electrode (DME) or a static mercury drop electrode (SMDE), which are useful for their wide cathodic ranges and renewable surfaces. It was invented in 1922 by Czechoslovak chemist Jaroslav Heyrovský, for which he won the Nobel prize in 1959. The main advantages of mercury as electrode material are as follows: 1) a large voltage window: ca. from +0.2 V to -1.8 V vs reversible hydrogen electrode (RHE). Hg electrode is particularly well-suited for studying electroreduction reactions. 2) very reproducible electrode surface, since mercury is liquid. 3) very easy cleaning of the electrode surface by making a new drop of mercury from a large Hg pool connected by a glass capillary.

A voltameter or coulometer is a scientific instrument used for measuring electric charge through electrolytic action. The SI unit of electric charge is the coulomb.

An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its negative terminal is the anode. The terminal marked negative is the source of electrons that will flow through an external electric circuit to the positive terminal. When a battery is connected to an external electric load, a redox reaction converts high-energy reactants to lower-energy products, and the free-energy difference is delivered to the external circuit as electrical energy. Historically the term "battery" specifically referred to a device composed of multiple cells; however, the usage has evolved to include devices composed of a single cell.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
If an electric field is applied parallel to the surface of a liquid and this surface has a net charge then the surface and so the liquid will move in response to the field. This is electrocapillary flow, an example of electrocapillarity. Electrocapillary phenomena are phenomena related to changes in the surface free energy of charged fluid interfaces, for example that of the dropping mercury electrode (DME), or in principle, any electrode, as the electrode potential changes or the electrolytic solution composition and concentration change.

In chemical analysis, capillary electrochromatography (CEC) is a chromatographic technique in which the mobile phase is driven through the chromatographic bed by electro-osmosis. Capillary electrochromatography is a combination of two analytical techniques, high-performance liquid chromatography and capillary electrophoresis. Capillary electrophoresis aims to separate analytes on the basis of their mass-to-charge ratio by passing a high voltage across ends of a capillary tube, which is filled with the analyte. High-performance liquid chromatography separates analytes by passing them, under high pressure, through a column filled with stationary phase. The interactions between the analytes and the stationary phase and mobile phase lead to the separation of the analytes. In capillary electrochromatography capillaries, packed with HPLC stationary phase, are subjected to a high voltage. Separation is achieved by electrophoretic migration of solutes and differential partitioning.

Electrochemical quartz crystal microbalance (EQCM) is the combination of electrochemistry and quartz crystal microbalance, which was generated in the eighties. Typically, an EQCM device contains an electrochemical cells part and a QCM part. Two electrodes on both sides of the quartz crystal serve two purposes. Firstly, an alternating electric field is generated between the two electrodes for making up the oscillator. Secondly, the electrode contacting electrolyte is used as a working electrode (WE), together with a counter electrode (CE) and a reference electrode (RE), in the potentiostatic circuit constituting the electrochemistry cell. Thus, the working electrode of electrochemistry cell is the sensor of QCM.
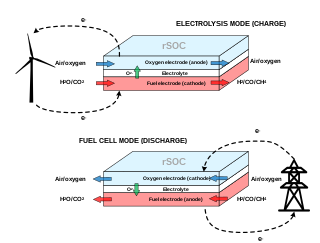
A reversible solid oxide cell (rSOC) is a solid-state electrochemical device that is operated alternatively as a solid oxide fuel cell (SOFC) and a solid oxide electrolysis cell (SOEC). Similarly to SOFCs, rSOCs are made of a dense electrolyte sandwiched between two porous electrodes. Their operating temperature ranges from 600°C to 900°C, hence they benefit from enhanced kinetics of the reactions and increased efficiency with respect to low-temperature electrochemical technologies.















