Related Research Articles
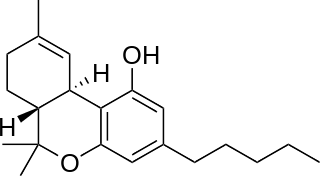
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term THC usually refers to the Delta-9-THC isomer with chemical name (−)-trans-Δ9-tetrahydrocannabinol. THC is a terpenoid found in cannabis and, like many pharmacologically active phytochemicals, it is assumed to be involved in the plant's evolutionary adaptation against insect predation, ultraviolet light, and environmental stress. THC was first discovered and isolated by Israeli chemist Raphael Mechoulam in Israel in 1964. It was found that, when smoked, THC is absorbed into the bloodstream and travels to the brain, attaching itself to endocannabinoid receptors located in the cerebral cortex, cerebellum, and basal ganglia. These are the parts of the brain responsible for thinking, memory, pleasure, coordination and movement.

Cannabinoids are several structural classes of compounds found in the cannabis plant primarily and most animal organisms or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary psychoactive compound in cannabis. Cannabidiol (CBD) is also a major constituent of temperate cannabis plants and a minor constituent in tropical varieties. At least 113 distinct phytocannabinoids have been isolated from cannabis, although only four have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea.

Secondary metabolites, also called specialised metabolites, toxins, secondary products, or natural products, are organic compounds produced by any lifeform, e.g. bacteria, fungi, animals, or plants, which are not directly involved in the normal growth, development, or reproduction of the organism. Instead, they generally mediate ecological interactions, which may produce a selective advantage for the organism by increasing its survivability or fecundity. Specific secondary metabolites are often restricted to a narrow set of species within a phylogenetic group. Secondary metabolites often play an important role in plant defense against herbivory and other interspecies defenses. Humans use secondary metabolites as medicines, flavourings, pigments, and recreational drugs.

A biogenic substance is a product made by or of life forms. While the term originally was specific to metabolite compounds that had toxic effects on other organisms, it has developed to encompass any constituents, secretions, and metabolites of plants or animals. In context of molecular biology, biogenic substances are referred to as biomolecules. They are generally isolated and measured through the use of chromatography and mass spectrometry techniques. Additionally, the transformation and exchange of biogenic substances can by modelled in the environment, particularly their transport in waterways.

In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered.

Pharmacognosy is the study of crude drugs obtained from medicinal plants, animals, fungi, and other natural sources. The American Society of Pharmacognosy defines pharmacognosy as "the study of the physical, chemical, biochemical, and biological properties of drugs, drug substances, or potential drugs or drug substances of natural origin as well as the search for new drugs from natural sources".

Cannabis sativa is an annual herbaceous flowering plant indigenous to Eastern Asia, but now of cosmopolitan distribution due to widespread cultivation. It has been cultivated throughout recorded history, used as a source of industrial fiber, seed oil, food, recreation, religious and spiritual states and medicine. Each part of the plant is harvested differently, depending on the purpose of its use. The species was first classified by Carl Linnaeus in 1753. The word sativa means "things that are cultivated."

A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical synthesis and have played a central role in the development of the field of organic chemistry by providing challenging synthetic targets. The term natural product has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients.
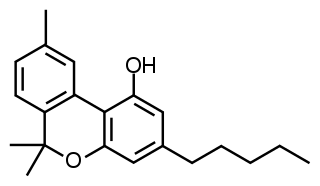
Cannabinol (CBN) is a mildly psychoactive cannabinoid that acts as a low affinity partial agonist at both CB1 and CB2 receptors. This activity at CB1 and CB2 receptors constitutes interaction of CBN with the endocannabinoid system (ECS).
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds containing at least one covalently bonded atom of chlorine. The chloroalkane class includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious.
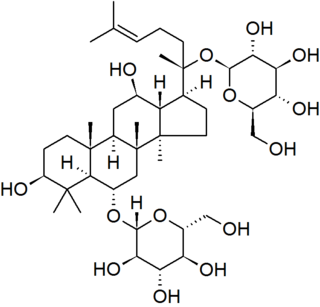
Ginsenosides or panaxosides are a class of natural product steroid glycosides and triterpene saponins. Compounds in this family are found almost exclusively in the plant genus Panax (ginseng), which has a long history of use in traditional medicine that has led to the study of pharmacological effects of ginseng compounds. As a class, ginsenosides exhibit a large variety of subtle and difficult-to-characterize biological effects when studied in isolation.

A depside is a type of polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester group. Depsides are most often found in lichens, but have also been isolated from higher plants, including species of the Ericaceae, Lamiaceae, Papaveraceae and Myrtaceae.

Sargassum muticum, commonly known as Japanese wireweed or japweed, is a large brown seaweed of the genus Sargassum. It is an invasive seaweed with high growth rate. It has an efficient dispersion thanks to its floats.

In biochemistry, naturally occurring phenols are natural products containing at least one phenol functional group. Phenolic compounds are produced by plants and microorganisms. Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research. Some phenols are germicidal and are used in formulating disinfectants.

Chemical defense is a strategy employed by many organisms to avoid consumption by producing toxic or repellent metabolites or chemical warnings which incite defensive behavioral changes. The production of defensive chemicals occurs in plants, fungi, and bacteria, as well as invertebrate and vertebrate animals. The class of chemicals produced by organisms that are considered defensive may be considered in a strict sense to only apply to those aiding an organism in escaping herbivory or predation. However, the distinction between types of chemical interaction is subjective and defensive chemicals may also be considered to protect against reduced fitness by pests, parasites, and competitors. Repellent rather than toxic metabolites are allomones, a sub category signaling metabolites known as semiochemicals. Many chemicals used for defensive purposes are secondary metabolites derived from primary metabolites which serve a physiological purpose in the organism. Secondary metabolites produced by plants are consumed and sequestered by a variety of arthropods and, in turn, toxins found in some amphibians, snakes, and even birds can be traced back to arthropod prey. There are a variety of special cases for considering mammalian antipredatory adaptations as chemical defenses as well.

Salinispora is a genus of obligately aerobic, gram-positive, non-acid-fast bacteria belonging to the family of Micromonosporaceae. They are heterotrophic, non-motile, and obligately grow under high osmotic/ionic-strength conditions. They are the first identified genus of gram-positive bacteria which has a high osmotic/ionic-strength requirement for survival. They are widely abundant in tropical marine sediments and were first identified in 2002. This genus of bacteria has potential biotechnological significance due to their production of novel secondary metabolites which can be used pharmaceutically.

Cannabidiolic acid (CBDA), is a cannabinoid found in cannabis plants. It is most abundant in the glandular trichomes on the female seedless flowers or more accurately infructescence often colloquially referred to as buds. CBDA is the chemical precursor to cannabidiol (CBD). Through the process of decarboxylation cannabidiol is derived via a loss of a carbon and two oxygen atoms from the 1 position of the benzoic acid ring. Cannabinoids are a class of compounds that are essentially unique to the cannabis genus. Both marijuana and hemp belong to this genus.

Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC, but for the first time in 2019 was isolated as a natural product in trace amounts from Cannabis sativa. It is structurally similar to Δ9-THC, the main active component of cannabis, but with the pentyl side chain extended to heptyl. Since it has a longer side chain, its cannabinoid effects are "far higher than Δ9-THC itself." Tetrahydrocannabiphorol has a reported binding affinity of 1.2 nM at CB1, approximately 33 times that of Δ9-THC (40 nM at CB1).

Hexahydrocannabinol (HHC) is a hydrogenated derivative of tetrahydrocannabinol (THC). It is a naturally occurring phytocannabinoid that has rarely been identified as a trace component in Cannabis sativa, but can also be produced synthetically by hydrogenation of cannabis extracts. The synthesis and bioactivity of HHC was first reported in 1940 by Roger Adams using tetrahydrocannabinol prepared from cannabidiol.
Cannabinoids are compounds found in the cannabis plant or synthetic compounds that can interact with the endocannabinoid system. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (Delta-9-THC), the primary intoxicating compound in cannabis. Cannabidiol (CBD) is another major constituent of some cannabis plants. At least 113 distinct cannabinoids have been isolated from cannabis.
References
- ↑ Page, J. E.; Nagel, J. (2006). "Chapter eight Biosynthesis of terpenophenolic metabolites in hop and cannabis". Integrative Plant Biochemistry. Recent Advances in Phytochemistry. Vol. 40. p. 179. doi:10.1016/S0079-9920(06)80042-0. ISBN 9780080451251.
- ↑ Simon-Levert, A.; Menniti, C.; Soulère, L.; Genevière, A. M.; Barthomeuf, C.; Banaigs, B.; Witczak, A. (2010). "Marine Natural Meroterpenes: Synthesis and Antiproliferative Activity". Marine Drugs. 8 (2): 347–358. doi: 10.3390/md8020347 . PMC 2852842 . PMID 20390109.