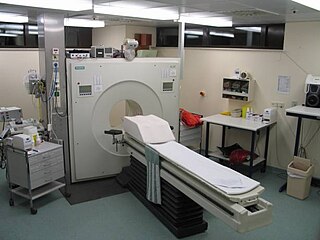
Positron-emission tomography (PET) is a nuclear medicine functional imaging technique that is used to observe metabolic processes in the body as an aid to the diagnosis of disease. The system detects pairs of gamma rays emitted indirectly by a positron-emitting radioligand, most commonly fluorine-18, which is introduced into the body on a biologically active molecule called a radioactive tracer. Different ligands are used for different imaging purposes, depending on what the radiologist/researcher wants to detect. Three-dimensional images of tracer concentration within the body are then constructed by computer analysis. In modern PET computed tomography scanners, three-dimensional imaging is often accomplished with the aid of a computed tomography X-ray scan performed on the patient during the same session, in the same machine.
A radioactive tracer, radiotracer, or radioactive label, is a chemical compound in which one or more atoms have been replaced by a radionuclide so by virtue of its radioactive decay it can be used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products. Radiolabeling or radiotracing is thus the radioactive form of isotopic labeling.

Scintigraphy, also known as a gamma scan, is a diagnostic test in nuclear medicine, where radioisotopes attached to drugs that travel to a specific organ or tissue (radiopharmaceuticals) are taken internally and the emitted gamma radiation is captured by external detectors to form two-dimensional images in a similar process to the capture of x-ray images. In contrast, SPECT and positron emission tomography (PET) form 3-dimensional images, and are therefore classified as separate techniques to scintigraphy, although they also use gamma cameras to detect internal radiation. Scintigraphy is unlike a diagnostic X-ray where external radiation is passed through the body to form an image.

Radiopharmacology is radiochemistry applied to medicine and thus the pharmacology of radiopharmaceuticals. Radiopharmaceuticals are used in the field of nuclear medicine as radioactive tracers in medical imaging and in therapy for many diseases. Many radiopharmaceuticals use technetium-99m (Tc-99m) which has many useful properties as a gamma-emitting tracer nuclide. In the book Technetium a total of 31 different radiopharmaceuticals based on Tc-99m are listed for imaging and functional studies of the brain, myocardium, thyroid, lungs, liver, gallbladder, kidneys, skeleton, blood and tumors.

Fluorodeoxyglucose (18F) (INN), or fluorodeoxyglucose F 18, also commonly called fluorodeoxyglucose and abbreviated [18F]FDG, 18F-FDG or FDG, is a radiopharmaceutical used in the medical imaging modality positron emission tomography (PET). Chemically, it is 2-deoxy-2-[18F]fluoro-D-glucose, a glucose analog, with the positron-emitting radionuclide fluorine-18 substituted for the normal hydroxyl group at the C-2 position in the glucose molecule.
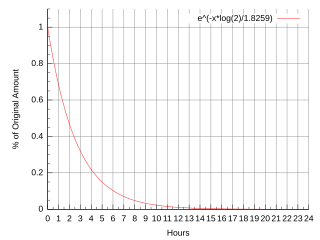
Fluorine-18 (18F) is a fluorine radioisotope which is an important source of positrons. It has a mass of 18.0009380(6) u and its half-life is 109.771(20) minutes. It decays by positron emission 97% of the time and electron capture 3% of the time. Both modes of decay yield stable oxygen-18.
A gallium scan is a type of nuclear medicine test that uses either a gallium-67 (67Ga) or gallium-68 (68Ga) radiopharmaceutical to obtain images of a specific type of tissue, or disease state of tissue. Gallium salts like gallium citrate and gallium nitrate may be used. The form of salt is not important, since it is the freely dissolved gallium ion Ga3+ which is active. Both 67Ga and 68Ga salts have similar uptake mechanisms. Gallium can also be used in other forms, for example 68Ga-PSMA is used for cancer imaging. The gamma emission of gallium 67 is imaged by a gamma camera, while the positron emission of gallium 68 is imaged by positron emission tomography (PET).

2-Deoxy-d-glucose is a glucose molecule which has the 2-hydroxyl group replaced by hydrogen, so that it cannot undergo further glycolysis. As such; it acts to competitively inhibit the production of glucose-6-phosphate from glucose at the phosphoglucoisomerase level. In most cells, glucose hexokinase phosphorylates 2-deoxyglucose, trapping the product 2-deoxyglucose-6-phosphate intracellularly ; thus, labelled forms of 2-deoxyglucose serve as a good marker for tissue glucose uptake and hexokinase activity. Many cancers have elevated glucose uptake and hexokinase levels. 2-Deoxyglucose labeled with tritium or carbon-14 has been a popular ligand for laboratory research in animal models, where distribution is assessed by tissue-slicing followed by autoradiography, sometimes in tandem with either conventional or electron microscopy.

Radioactivity is generally used in life sciences for highly sensitive and direct measurements of biological phenomena, and for visualizing the location of biomolecules radiolabelled with a radioisotope.
Joanna Sigfred Fowler is a scientist emeritus at the U.S. Department of Energy's Brookhaven National Laboratory in New York. She served as professor of psychiatry at Mount Sinai School of Medicine and director of Brookhaven's Radiotracer Chemistry, Instrumentation and Biological Imaging Program. Fowler studied the effect of disease, drugs, and aging on the human brain and radiotracers in brain chemistry. She has received many awards for her pioneering work, including the National Medal of Science.
Copper-64 (64Cu) is a positron emitting isotope of copper, with applications for molecular radiotherapy and positron emission tomography.
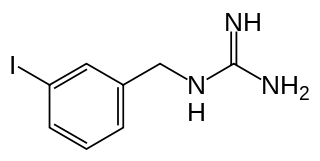
Iobenguane, or MIBG, is an aralkylguanidine analog of the adrenergic neurotransmitter norepinephrine and a radiopharmaceutical. It acts as a blocking agent for adrenergic neurons. When radiolabeled, it can be used in nuclear medicinal diagnostic techniques as well as in neuroendocrine antineoplastic treatments.

Brain positron emission tomography is a form of positron emission tomography (PET) that is used to measure brain metabolism and the distribution of exogenous radiolabeled chemical agents throughout the brain. PET measures emissions from radioactively labeled metabolically active chemicals that have been injected into the bloodstream. The emission data from brain PET are computer-processed to produce multi-dimensional images of the distribution of the chemicals throughout the brain.

Radiopharmaceuticals, or medicinal radiocompounds, are a group of pharmaceutical drugs containing radioactive isotopes. Radiopharmaceuticals can be used as diagnostic and therapeutic agents. Radiopharmaceuticals emit radiation themselves, which is different from contrast media which absorb or alter external electromagnetism or ultrasound. Radiopharmacology is the branch of pharmacology that specializes in these agents.
Fluorodeoxyglycosylamine is a product of fluorodeoxyglucose and biological amines. The Maillard reaction of sugars and amines results in the formation of glycosylamines and Amadori products that are of biological significance, for drug delivery, role in central nervous system, and other potential applications.

PET radiotracer is a type of radioligand that is used for the diagnostic purposes via positron emission tomography imaging technique.

Fluorothymidine F-18 (FLT) is a tumor-specific PET tracer and radiopharmaceutical. and a isotopologue of alovudine. FLT is suitable for monitoring how tumors respond to cytostatic therapy. FLT accumulates in proliferating cells where it indicates the activity of the enzyme thymidine kinase. Cell division can be characterized by the activity of that enzyme. FLT is phosphorylated as though it were thymidine, and is subsequently incorporated into DNA. Thymidine is essential for DNA replication. Considering that FLT lacks a 3′-hydroxy group, transcription of DNA is impeded following incorporating of FLT. FLT indicates changes in tumor cell proliferation by tracking the restoration of nucleosides from degenerated DNA.

Fluciclovine (18F), also known as anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid (FACBC), or as Axumin, and colloquially as anti-3[18F] FACBC or F18, is a diagnostic agent "indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated prostate specific antigen (PSA) levels."

Carbon-11 choline is the basis of medical imaging technologies. Because of its involvement in biologic processes, choline is related to diseases, leading to the development of medical imaging techniques to monitor its concentration. When radiolabeled with 11CH3, choline is a useful a tracer in PET imaging. Carbon-11 is radioactive with a half-life of 20.38 minutes. By monitoring the gamma radiation resulting from the decay of carbon-11, the uptake, distribution, and retention of carbon-11 choline can be monitored.

Oxygen-15 labelled water (also known as 15O-water, [O-15]-H2O, or H215O) is a radioactive variation of regular water, in which the oxygen atom has been replaced by oxygen-15 (15O), a positron-emitting isotope. 15O-water is used as a radioactive tracer for measuring and quantifying blood flow using positron emission tomography (PET) in the heart, brain and tumors.













