Related Research Articles

Adenosine triphosphate (ATP) is a nucleotide that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, it is often referred to as the "molecular unit of currency" of intracellular energy transfer.

The citric acid cycle—also known as the Krebs cycle, Szent-Györgyi-Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of biochemical reactions to release the energy stored in nutrients through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The chemical energy released is available under the form of ATP. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.

Glycolysis is the metabolic pathway that converts glucose into pyruvate and, in most organisms, occurs in the liquid part of cells. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.
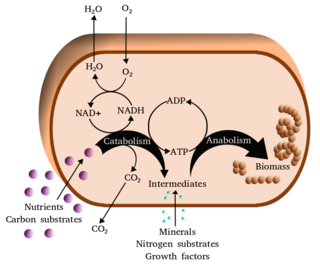
Metabolism is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the conversion of food to building blocks of proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to the sum of all chemical reactions that occur in living organisms, including digestion and the transportation of substances into and between different cells, in which case the above described set of reactions within the cells is called intermediary metabolism.

Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it (or a thioester) as a substrate. In humans, CoA biosynthesis requires cysteine, pantothenate (vitamin B5), and adenosine triphosphate (ATP).
Pyruvic acid (IUPAC name: 2-oxopropanoic acid, also called acetoic acid) (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.

Cellular respiration is the process by which biological fuels are oxidized in the presence of an inorganic electron acceptor, such as oxygen, to drive the bulk production of adenosine triphosphate (ATP), which contains energy. Cellular respiration may be described as a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into ATP, and then release waste products.

Acetyl-CoA is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle to be oxidized for energy production.
The term amphibolic is used to describe a biochemical pathway that involves both catabolism and anabolism. Catabolism is a degradative phase of metabolism in which large molecules are converted into smaller and simpler molecules, which involves two types of reactions. First, hydrolysis reactions, in which catabolism is the breaking apart of molecules into smaller molecules to release energy. Examples of catabolic reactions are digestion and cellular respiration, where sugars and fats are broken down for energy. Breaking down a protein into amino acids, or a triglyceride into fatty acids, or a disaccharide into monosaccharides are all hydrolysis or catabolic reactions. Second, oxidation reactions involve the removal of hydrogens and electrons from an organic molecule. Anabolism is the biosynthesis phase of metabolism in which smaller simple precursors are converted to large and complex molecules of the cell. Anabolism has two classes of reactions. The first are dehydration synthesis reactions; these involve the joining of smaller molecules together to form larger, more complex molecules. These include the formation of carbohydrates, proteins, lipids and nucleic acids. The second are reduction reactions, in which hydrogens and electrons are added to a molecule. Whenever that is done, molecules gain energy.

In the mitochondrion, the matrix is the space within the inner membrane. The word "matrix" stems from the fact that this space is viscous, compared to the relatively aqueous cytoplasm. The mitochondrial matrix contains the mitochondrial DNA, ribosomes, soluble enzymes, small organic molecules, nucleotide cofactors, and inorganic ions.[1] The enzymes in the matrix facilitate reactions responsible for the production of ATP, such as the citric acid cycle, oxidative phosphorylation, oxidation of pyruvate, and the beta oxidation of fatty acids.
Fatty acid metabolism consists of various metabolic processes involving or closely related to fatty acids, a family of molecules classified within the lipid macronutrient category. These processes can mainly be divided into (1) catabolic processes that generate energy and (2) anabolic processes where they serve as building blocks for other compounds.
In biochemistry, lipogenesis is the conversion of fatty acids and glycerol into fats, or a metabolic process through which acetyl-CoA is converted to triglyceride for storage in fat. Lipogenesis encompasses both fatty acid and triglyceride synthesis, with the latter being the process by which fatty acids are esterified to glycerol before being packaged into very-low-density lipoprotein (VLDL). Fatty acids are produced in the cytoplasm of cells by repeatedly adding two-carbon units to acetyl-CoA. Triacylglycerol synthesis, on the other hand, occurs in the endoplasmic reticulum membrane of cells by bonding three fatty acid molecules to a glycerol molecule. Both processes take place mainly in liver and adipose tissue. Nevertheless, it also occurs to some extent in other tissues such as the gut and kidney. A review on lipogenesis in the brain was published in 2008 by Lopez and Vidal-Puig. After being packaged into VLDL in the liver, the resulting lipoprotein is then secreted directly into the blood for delivery to peripheral tissues.
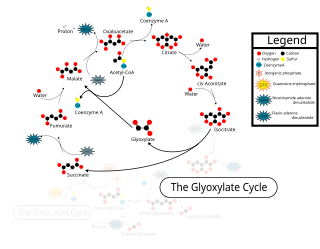
The glyoxylate cycle, a variation of the tricarboxylic acid cycle, is an anabolic pathway occurring in plants, bacteria, protists, and fungi. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of carbohydrates. In microorganisms, the glyoxylate cycle allows cells to use two carbons, such as acetate, to satisfy cellular carbon requirements when simple sugars such as glucose or fructose are not available. The cycle is generally assumed to be absent in animals, with the exception of nematodes at the early stages of embryogenesis. In recent years, however, the detection of malate synthase (MS) and isocitrate lyase (ICL), key enzymes involved in the glyoxylate cycle, in some animal tissue has raised questions regarding the evolutionary relationship of enzymes in bacteria and animals and suggests that animals encode alternative enzymes of the cycle that differ in function from known MS and ICL in non-metazoan species.
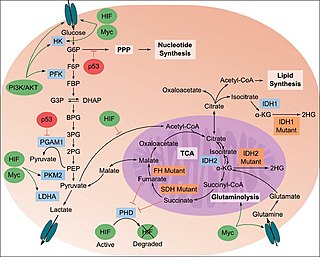
The study of the tumor metabolism, also known as tumor metabolome describes the different characteristic metabolic changes in tumor cells. The characteristic attributes of the tumor metabolome are high glycolytic enzyme activities, the expression of the pyruvate kinase isoenzyme type M2, increased channeling of glucose carbons into synthetic processes, such as nucleic acid, amino acid and phospholipid synthesis, a high rate of pyrimidine and purine de novo synthesis, a low ratio of Adenosine triphosphate and Guanosine triphosphate to Cytidine triphosphate and Uridine triphosphate, low Adenosine monophosphate levels, high glutaminolytic capacities, release of immunosuppressive substances and dependency on methionine.
Propionyl-CoA is a coenzyme A derivative of propionic acid. It is composed of a 24 total carbon chain and its production and metabolic fate depend on which organism it is present in. Several different pathways can lead to its production, such as through the catabolism of specific amino acids or the oxidation of odd-chain fatty acids. It later can be broken down by propionyl-CoA carboxylase or through the methylcitrate cycle. In different organisms, however, propionyl-CoA can be sequestered into controlled regions, to alleviate its potential toxicity through accumulation. Genetic deficiencies regarding the production and breakdown of propionyl-CoA also have great clinical and human significance.

The glycosome is a membrane-enclosed organelle that contains the glycolytic enzymes. The term was first used by Scott and Still in 1968 after they realized that the glycogen in the cell was not static but rather a dynamic molecule. It is found in a few species of protozoa including the Kinetoplastida which include the suborders Trypanosomatida and Bodonina, most notably in the human pathogenic trypanosomes, which can cause sleeping sickness, Chagas's disease, and leishmaniasis. The organelle is bounded by a single membrane and contains a dense proteinaceous matrix. It is believed to have evolved from the peroxisome. This has been verified by work done on Leishmania genetics.
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is converted into fatty acids is derived from carbohydrates via the glycolytic pathway. The glycolytic pathway also provides the glycerol with which three fatty acids can combine to form triglycerides, the final product of the lipogenic process. When only two fatty acids combine with glycerol and the third alcohol group is phosphorylated with a group such as phosphatidylcholine, a phospholipid is formed. Phospholipids form the bulk of the lipid bilayers that make up cell membranes and surrounds the organelles within the cells. In addition to cytosolic fatty acid synthesis, there is also mitochondrial fatty acid synthesis (mtFASII), in which malonyl-CoA is formed from malonic acid with the help of malonyl-CoA synthetase (ACSF3), which then becomes the final product octanoyl-ACP (C8) via further intermediate steps.
The Randle cycle, also known as the glucose fatty-acid cycle, is a metabolic process involving the competition of glucose and fatty acids for substrates. It is theorized to play a role in explaining type 2 diabetes and insulin resistance.
Glutaminolysis (glutamine + -lysis) is a series of biochemical reactions by which the amino acid glutamine is lysed to glutamate, aspartate, CO2, pyruvate, lactate, alanine and citrate.
Metabolite channeling is the passing of the intermediary metabolic product of one enzyme directly to another enzyme or active site without its release into solution. When several consecutive enzymes of a metabolic pathway channel substrates between themselves, this is called a metabolon. Channeling can make a metabolic pathway more rapid and efficient than it would be if the enzymes were randomly distributed in the cytosol, or prevent the release of unstable intermediates. It can also protect an intermediate from being consumed by competing reactions catalyzed by other enzymes.
References
- ↑ Wu, Fei; Minteer, Shelley (2 February 2015). "Krebs Cycle Metabolon: Structural Evidence of Substrate Channeling Revealed by Cross-Linking and Mass Spectrometry". Angewandte Chemie International Edition. 54 (6): 1851–1854. doi: 10.1002/anie.201409336 . PMID 25537779.
- ↑ Zhang, Youjun; Beard, Katherine F. M.; Swart, Corné; Bergmann, Susan; Krahnert, Ina; Nikoloski, Zoran; Graf, Alexander; Ratcliffe, R. George; Sweetlove, Lee J.; Fernie, Alisdair R.; Obata, Toshihiro (16 May 2017). "Protein-protein interactions and metabolite channelling in the plant tricarboxylic acid cycle". Nature Communications. 8: 15212. doi:10.1038/ncomms15212. PMC 5440813 . PMID 28508886.
- ↑ Laursen, Tomas; Borch, Jonas; Knudsen, Camilla; Bavishi, Krutika; Torta, Federico; Martens, Helle Juel; Silvestro, Daniele; Hatzakis, Nikos S.; Wenk, Markus R. (2016-11-18). "Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum" (PDF). Science. 354 (6314): 890–893. doi:10.1126/science.aag2347. ISSN 0036-8075. PMID 27856908. S2CID 19187608.
- ↑ Kuzin A. M. Structural – metabolic hypothesis in radiobiology. Moscow: Nauka Ed., 1970.- 50 p.
- ↑ Srere P. A. Is there an organization of Krebs cycle enzymes in the mitochondrial matrix? In: Energy Metabolism and the Regulation of Metabolic Processes in Mitochondria, R. W. Hanson and W.A. Mehlman (Eds.). New York: Academic Press. 1972. p.79-91.
- ↑ Lyubarev, A. E.; Kurganov, B. I. (1989). "Supramolecular organization of tricarboxylic acid cycle enzymes". Biosystems. 22 (2): 91–102. doi:10.1016/0303-2647(89)90038-5. PMID 2720141.
- ↑ Lyubarev A. E., Kurganov B. I. Supramolecular organisation of Tricarboxylic Acids Cycle's enzymes. Proceedings of the All-Union Symposium "Molecular mechanisms and regulation of energy metabolism". Puschino, Russia, 1986. p. 13. (in Russian) .
- ↑ Kurganov B. I, Lyubarev A. E. Hypothetical structure of the complex of glycolytic enzymes (glycolytic metabolon), formed on the membrane of erythrocytes. Molek. Biologia. 1988. V.22, No.6, p. 1605–1613. (in Russian)
- ↑ Kurganov B.I., Lyubarev A.E. Enzymes and multienzyme complexes as controllable systems. In: Soviet Scientific Reviews. Section D. Physicochemical Biology Reviews. V. 8 (ed. V.P. Skulachev). Glasgow, Harwood Acad. Publ., 1988, p. 111-147
- ↑ Clarke, F. M.; Masters, C. J. (1975). "On the association of glycolytic enzymes with structural proteins of skeletal muscle". Biochimica et Biophysica Acta (BBA) - General Subjects. 381 (1): 37–46. doi:10.1016/0304-4165(75)90187-7. PMID 1111588.
- ↑ Clarke, F. M.; Stephan, P.; Huxham, G.; Hamilton, D.; Morton, D. J. (1984). "Metabolic dependence of glycolytic enzyme binding in rat and sheep heart". European Journal of Biochemistry. 138 (3): 643–9. doi:10.1111/j.1432-1033.1984.tb07963.x. PMID 6692839.
- ↑ Srere, P. A. (1985). "The metabolon". Trends in Biochemical Sciences. 10 (3): 109–110. doi:10.1016/0968-0004(85)90266-X.
- ↑ Robinson, J. B., Jr. & Srere, P. A. (1986) Interactions of sequential metabolic enzymes of the mitochondria: a role in metabolic regulation, pp. 159–171 in Dynamics of Biochemical Systems (ed. Damjanovich, S., Keleti, T. & Trón, L.), Akadémiai Kiadó, Budapest, Hungary
- ↑ Kastritis, Panagiotis L.; O'Reilly, Francis J.; Bock, Thomas; Li, Yuanyue; Rogon, Matt Z.; Buczak, Katarzyna; Romanov, Natalie; Betts, Matthew J.; Bui, Khanh Huy (2017-07-01). "Capturing protein communities by structural proteomics in a thermophilic eukaryote". Molecular Systems Biology. 13 (7): 936. doi:10.15252/msb.20167412. ISSN 1744-4292. PMC 5527848 . PMID 28743795.
- ↑ Veliky M.M., Starikovich L. S., Klimishin N. I., Chayka Ya. P. Molecular mechanisms in the integration of metabolism. Lviv National University Ed., Lviv, Ukraine. 2007. 229 P. (in ukrainian) ISBN 978-966-613-538-7