Related Research Articles
Peptoids, or poly-N-substituted glycines, are a class of biochemicals known as biomimetics that replicate the behavior of biological molecules. Peptidomimetics are recognizable by side chains that are appended to the nitrogen atom of the peptide backbone, rather than to the α-carbons.

Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antibiotics which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators.
Cathelicidin antimicrobial peptide (CAMP) is a polypeptide that is primarily stored in the lysosomes of macrophages and polymorphonuclear leukocytes (PMNs); in humans, the CAMP gene encodes the peptide precursor CAP-18, which is processed by proteinase 3-mediated extracellular cleavage into the active form LL-37. LL-37 is the only peptide in the Cathelicidin family found in the human body.
Dermaseptins are a family of peptides isolated from skin of the frog genus Phyllomedusa.

Beta-defensin 1 is a protein that in humans is encoded by the DEFB1 gene.
Lingual antimicrobial peptide (LAP) is a beta-defensin found in bovine internal epithelial tissue, in particular, that of the digestive tract. It has antimicrobial activity against many different pathogens. It was first isolated from an inflamed cattle tongue, hence its designation as lingual. Since then it has been found more extensively throughout the body; its presence has even been detected in bovine milk. Its expression is selective and increases in inflamed areas. LAP may have a closer relationship with immune response than simple antimicrobial activity, such as an association with growth factor activity.
Pseudin is a peptide derived from Pseudis paradoxa. Pseudins have some antimicrobial function.
The magainins are a class of antimicrobial peptides found in the African clawed frog. The peptides are cationic, generally lack a stable conformation in water but form amphipathic α-helix in membranes; their mechanism against micro-organisms is unclear but they disrupt the cell membranes of a broad spectrum of bacteria, protozoa, and fungi.
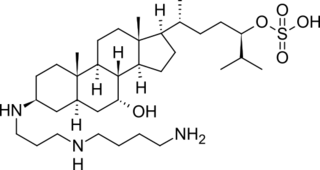
Squalamine is a steroid-polyamine conjugate compound with broad spectrum antimicrobial activity and anti-angiogenic activity. It was studied as a potential cancer drug and as a potential treatment for wet macular degeneration but as of 2018 had not succeeded in Phase III trials for any use.
The International Fibrodysplasia Ossificans Progressiva Association (IFOPA) is a US-based 501(c)(3) non-profit organization supporting medical research, education and communication for those afflicted by the rare genetic condition Fibrodysplasia Ossificans Progressiva (FOP). IFOPA's mission is to fund research to find a cure for FOP while supporting, connecting, and advocating for individuals with FOP and their families, and raising awareness worldwide. IFOPA is governed by a volunteer board of directors which may range in number from 9 to 15, at least one of whom must have FOP. The association's location is 1520 Clay St., Suite H2, North Kansas City, MO, 64116, part of the Kansas City, Missouri metropolitan area.
Arenicins are a group of antimicrobial peptides being studied to combat Gram-negative bacteria.
Brilacidin, an investigational new drug (IND), is a polymer-based antibiotic currently in human clinical trials, and represents a new class of antibiotics called host defense protein mimetics, or HDP-mimetics, which are non-peptide synthetic small molecules modeled after host defense peptides (HDPs). HDPs, also called antimicrobial peptides, some of which are defensins, are part of the innate immune response and are common to most higher forms of life. As brilacidin is modeled after a defensin, it is also called a defensin mimetic.
David Grainger is a partner at medicxi, a European life sciences-oriented venture capital firm and chief executive officer of Methuselah Health Ltd., a drug development company doing proteomics research in the longevity space.
Michael S. Gilmore is an American, focusing in infectious diseases and ocular genomics, currently the Sir William Osler Professor of Ophthalmology (Microbiology), Harvard Medical School, Mike serves as Director of the Infectious Disease Institute, and Co-Director of the Microbial Sciences Initiative of Harvard University. Additionally, he is a Senior Associate Member of the Broad Institute. As Principal Investigator of the Harvard-wide Program on Antibiotic Resistance, his research focuses on the evolution and development of multidrug resistant strains of enterococci, staphylococci, and streptococci, and the development of new therapeutics. He was named by Eric Lander in “The Heroes of CRISPR”3 as inspiring Broad Institute interest in developing CRISPR as a tool for therapeutic gene editing. Mike has trained over 35 graduate students and postdocs, and is currently course coordinator and principle lecturer in the Harvard University course OEB290/MICRO210 Microbiology: Chemistry, ecology and evolution. Outside of Harvard, he serves as chair of the US National Institutes of Health (NIH) blue ribbon panel for the Antimicrobial Resistance Diagnostic Challenge. He is past chair of the NIH Bacterial Pathogenesis Study Section, the Gordon Conference on Microbial Adhesion and Signal Transduction, American Society for Microbiology (ASM) Division D, and the Association for Research in Vision and Ophthalmology (ARVO) IM Section. Mike is founder of the International Conference on Enterococci (ICE) series, and the Boston Area Antibiotic Resistance Network (BAARN). He started his academic career in 1984 at the University of Oklahoma Health Sciences Center, where he rose through the ranks to Vice President for Research. He also held the MG McCool professorship and was awarded the George Lynn Cross research chair. In 2004 he moved to Harvard Medical School as President and CEO of the Schepens Eye Research Institute, Marie and DeWalt Ankeny Director of Research and CL Schepens Professor of Ophthalmology. In 2010, he moved his laboratories to the Massachusetts General Hospital campus, in the Massachusetts Eye and Ear Infirmary. He has published over 200 peer reviewed manuscripts in Cell, Nature, Science, PNAS and other leading journals. He continues to serve on numerous advisory boards and committees for public and private organizations, focused on drug discovery, antibiotic resistance, and bacterial pathogenesis.
Gregory L. Verdine is an American chemical biologist, biotech entrepreneur, venture capitalist and university professor. He is a founder of the field of chemical biology, which deals with the application of chemical techniques to biological systems. His work has focused on mechanisms of DNA repair and cell penetrability.
Esculentin-2CHa is an antimicrobial peptide located outside the epithelial cell's membrane of the skin of many species of amphibians, such as Rana chiricahuensis. This peptide has recently become more important due to its defense response function and its possible application in the treatment of various human pathologies, that range from type 2 diabetes to bacterial and fungi infections. Esculentin-2CHa is a peptide that belongs to the Esculentin-2 family, which is known for its broad-spectrum of antimicrobial activity and its low cytotoxicity to human erythrocytes. However, not much is known about its structures and their relation to the functions these peptides carry out.

James Inglese is an American biochemist, the director of the Assay Development and Screening Technology laboratory at the National Center for Advancing Translational Sciences, a Center within the National Institutes of Health. His specialty is small molecule high throughput screening. Inglese's laboratory develops methods and strategies in molecular pharmacology with drug discovery applications. The work of his research group and collaborators focuses on genetic and infectious disease-associated biology.

Robert Ernest William Hancock is a Canadian microbiologist and University of British Columbia Killam Professor of Microbiology and Immunology, an Associate Faculty Member of the Wellcome Trust Sanger Institute, and a Canada Research Chair in Health and Genomics.
Robert David Moir was an Australian-born medical research scientist who theorized that the over-accumulation of beta-amyloid, which had formed to protect the brain against microbes, aided the development of Alzheimer's disease in the human brain.
Baramicin (Bara) is an antimicrobial peptide gene of the fruit fly Drosophila melanogaster. Baramicin is a prominent element of the fly immune response: of the most abundant immune peptides detected in the fly hemolymph, the BaraA gene is responsible for 9 of the 24 peptides first described for their high concentrations after systemic infection. The name of the Baramicin gene was inspired by One Piece character “Buggy" and derives from the Japanese expression "Bara Bara", an onomatopoeia for things breaking apart, in reference to the Baramicin precursor breaking into multiple sub-peptides.
References
- 1 2 Okie, Susan (February 16, 1988). "A Man and His Frogs". The Washington Post.
- 1 2 Brower, Montgomery (17 August 1987). "The Case of the Frog That Healed Leads Dr. Michael Zasloff to a Medical Leap Ahead". People Magazine . New York, New York.
- 1 2 "Press release: MacroChem Corporation Announces Michael Zasloff, M.D., Ph.D. Joins Company's Scientific Advisory Board". MacroChem via BioSpace. October 17, 2007.
- 1 2 3 4 5 Maeder, Thomas (February 1998). "A Few Hundred People Turned To Bone". The Atlantic.
- ↑ Altman, Lawrence K. (4 August 1987). "Staying Ahead of Microbes: New Progress". The New York Times.
- ↑ Conlon, JM; Mechkarska, M; King, JD (1 May 2012). "Host-defense peptides in skin secretions of African clawed frogs (Xenopodinae, Pipidae)". General and Comparative Endocrinology. 176 (3): 513–8. doi:10.1016/j.ygcen.2011.10.010. PMID 22036891.
- ↑ George, John (April 29, 2009). "Biotech Genaera shutting down: Never brought drug to market". Philadelphia Business Journal.
- ↑ "Zasloff bio". Georgetown University. Retrieved 16 January 2018.
- ↑ Moore, A (February 2003). "The big and small of drug discovery. Biotech versus pharma: advantages and drawbacks in drug development". EMBO Reports. 4 (2): 114–7. doi:10.1038/sj.embor.embor748. PMC 1315844 . PMID 12612596.
- ↑ "Press release: GUMC Discovery Chosen for Time's Top 100 Scientific Findings". Georgetown. May 2, 2013.
- ↑ "Formula XO Company Profile: Valuation & Investors". PitchBook. Retrieved 16 January 2018.
- ↑ Corsano, Erica (October 16, 2014). "Russo's Illumai creates beauty without beastly chemicals". Boston Herald. p. 28.
- ↑ Taylor, Phil (July 17, 2017). "Backers put $12.7M behind Enterin's Parkinson's disease approach". FierceBiotech.