The flash point of a volatile material is the lowest temperature at which vapours of the material will ignite, when given an ignition source.

Synthetic oil is a lubricant consisting of chemical compounds that are artificially made. Synthetic lubricants can be manufactured using chemically modified petroleum components rather than whole crude oil, but can also be synthesized from other raw materials. The base material, however, is still overwhelmingly crude oil that is distilled and then modified physically and chemically. The actual synthesis process and composition of additives is generally a commercial trade secret and will vary among producers.

Solid fuel refers to various forms of solid material that can be burnt to release energy, providing heat and light through the process of combustion. Solid fuels can be contrasted with liquid fuels and gaseous fuels. Common examples of solid fuels include wood, charcoal, peat, coal, Hexamine fuel tablets, wood pellets, corn, wheat, rye, and other grains. Solid fuels are extensively used in rocketry as solid propellants. Solid fuels have been used throughout human history to create fire and solid fuel is still in widespread use throughout the world in the present day.
Cetane number is an indicator of the combustion speed of diesel fuel and compression needed for ignition. It is an inverse of the similar octane rating for gasoline. The CN is an important factor in determining the quality of diesel fuel, but not the only one; other measurements of diesel's quality include energy content, density, lubricity, cold-flow properties and sulphur content.

Jet fuel, aviation turbine fuel (ATF), or avtur, is a type of aviation fuel designed for use in aircraft powered by gas-turbine engines. It is colorless to straw-colored in appearance. The most commonly used fuels for commercial aviation are Jet A and Jet A-1, which are produced to a standardized international specification. The only other jet fuel commonly used in civilian turbine-engine powered aviation is Jet B, which is used for its enhanced cold-weather performance.

Petroleum coke, abbreviated coke or petcoke, is a final carbon-rich solid material that derives from oil refining, and is one type of the group of fuels referred to as cokes. Petcoke is the coke that, in particular, derives from a final cracking process—a thermo-based chemical engineering process that splits long chain hydrocarbons of petroleum into shorter chains—that takes place in units termed coker units. Stated succinctly, coke is the "carbonization product of high-boiling hydrocarbon fractions obtained in petroleum processing ." Petcoke is also produced in the production of synthetic crude oil (syncrude) from bitumen extracted from Canada’s oil sands and from Venezuela's Orinoco oil sands.
Graphite furnace atomic absorption spectroscopy (GFAAS) is a type of spectrometry that uses a graphite-coated furnace to vaporize the sample. Briefly, the technique is based on the fact that free atoms will absorb light at frequencies or wavelengths characteristic of the element of interest. Within certain limits, the amount of light absorbed can be linearly correlated to the concentration of analyte present. Free atoms of most elements can be produced from samples by the application of high temperatures. In GFAAS, samples are deposited in a small graphite or pyrolytic carbon coated graphite tube, which can then be heated to vaporize and atomize the analyte. The atoms absorb ultraviolet or visible light and make transitions to higher electronic energy levels. Applying the Beer-Lambert law directly in AA spectroscopy is difficult due to variations in the atomization efficiency from the sample matrix, and nonuniformity of concentration and path length of analyte atoms. Concentration measurements are usually determined from a working curve after calibrating the instrument with standards of known concentration. The main advantages of the graphite furnace comparing to aspiration atomic absorption are the following:
In the petroleum industry, cloud point refers to the temperature below which wax in diesel or biowax in biodiesels forms a cloudy appearance. The presence of solidified waxes thickens the oil and clogs fuel filters and injectors in engines. The wax also accumulates on cold surfaces and forms an emulsion with water. Therefore, cloud point indicates the tendency of the oil to plug filters or small orifices at cold operating temperatures.
Coal analysis techniques are specific analytical methods designed to measure the particular physical and chemical properties of coals. These methods are used primarily to determine the suitability of coal for coking, power generation or for iron ore smelting in the manufacture of steel.
Ramsbottom carbon residue, which abbreviation is RCR, is well known in the petroleum industry as a method to calculate the carbon residue of a fuel. The carbon residue value is considered by some to give an approximate indication of the combustibility and deposit forming tendencies of the fuel.
A gas pycnometer is a laboratory device used for measuring the density—or, more accurately, the volume—of solids, be they regularly shaped, porous or non-porous, monolithic, powdered, granular or in some way comminuted, employing some method of gas displacement and the volume:pressure relationship known as Boyle's Law. A gas pycnometer is also sometimes referred to as a helium pycnometer.
Reid vapor pressure (RVP) is a common measure of the volatility of gasoline and other petroleum products. It is defined as the absolute vapor pressure exerted by the vapor of the liquid and any dissolved gases/moisture at 37.8 °C (100 °F) as determined by the test method ASTM-D-323, which was first developed in 1930 and has been revised several times. The test method measures the vapor pressure of gasoline, volatile crude oil, jet fuels, naphtha, and other volatile petroleum products but is not applicable for liquefied petroleum gases. ASTM D323-15a requires that the sample be chilled to 0-1 degrees Celsius and then poured into the apparatus; for any material that solidifies at this temperature, this step cannot be performed. RVP is commonly reported in kilopascals or pounds per square inch and represents volatization at atmospheric pressure because ASTM-D-323 measures the gauge pressure of the sample in a non-evacuated chamber.

Anton Paar ProveTec, formerly known as Petrotest GmbH, is a German company within the Anton Paar group that is known for its laboratory equipment for the chemical and petrochemical industries. Furthermore, Anton Paar ProveTec is manufacturing measurement instruments for the cosmetics industry, the aroma and fragrance industry, the food, and pharmaceutical industry. The company was founded in 1873 by Berthold Pensky, who also invented the Pensky-Martens flash point tester. The company is ISO-9001 certified and located south of Berlin in Dahlewitz.
Electron phenomenological spectroscopy (EPS) is based on the correlations between integral optical characteristics and properties of substance as a single whole quantum continuum: spectrum-properties and color-properties. According to these laws the physicochemical properties of substance solutions in ultraviolet (UV), visible light and near infrared (IR) regions of the electromagnetic spectrum are in proportion to the quantity of radiation absorbed. Such aspects of electron spectroscopy have been shown in the works of Mikhail Yu Dolomatov and has been named electron phenomenological spectroscopy because the integral characteristics of the system are studied. Qualitatively, new laws appear on the integral level.
A Cetane Improver [′sē‚tān im′prüv·ər] is a chemical which has the effect of increasing a diesel fuel's Cetane number. A few examples are nitrates, nitroalkanes, nitrocarbonates and peroxides.
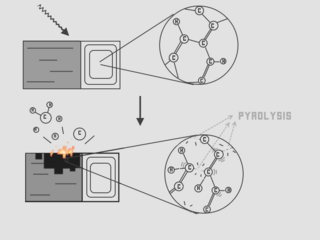
Conradson carbon residue, commonly known as "Concarbon" or "CCR" is a laboratory test used to provide an indication of the coke-forming tendencies of an oil. Quantitatively, the test measures the amount of carbonaceous residue remaining after the oil's evaporation and pyrolysis. In general, the test is applicable to petroleum products which are relatively non-volatile, and which decompose on distillation at atmospheric pressure. The phrase "Conradson carbon residue" and its common names can refer to either the test or the numerical value obtained from it.
Total Base Number (TBN) is a measurement of basicity that is expressed in terms of the equivalent number of milligrams of potassium hydroxide per gram of oil sample. TBN is an important measurement in petroleum products, and the value varies depending on its application. TBN generally ranges from 6–80 mg KOH/g in modern lubricants, 7–10 mg KOH/g for general internal combustion engine use and 10–15 mg KOH/g for diesel engine operations. TBN is typically higher for marine grade lubricants, approximately 15-80 mg KOH/g, as the higher TBN values are designed to increase the operating period under harsh operating conditions, before the lubricant requires replacement. it can be important measurement tool to determine purity of amines as well.