
In atomic theory and quantum mechanics, an atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term atomic orbital may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.

A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full outer shell, corresponding to a stable electronic configuration. In organic chemistry covalent bonds are much more common than ionic bonds.
A sports governing body is a sports organization that has a regulatory or sanctioning function. Sports governing bodies come in various forms, and have a variety of regulatory functions. Examples of this can include disciplinary action for rule infractions and deciding on rule changes in the sport that they govern. Governing bodies have different scopes. They may cover a range of sport at an International level, such as the International Olympic Committee and the International Paralympic Committee, or only a single sport at a national level, such as the Rugby Football League. National bodies will largely have to be affiliated to international bodies for the same sport. The first international federations were formed at the end of the 19th century.

Orbital inclination measures the tilt of an object's orbit around a celestial body. It is expressed as the angle between a reference plane and the orbital plane or axis of direction of the orbiting object.

In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using the notation explained below.

Orbital mechanics or astrodynamics is the application of ballistics and celestial mechanics to the practical problems concerning the motion of rockets and other spacecraft. The motion of these objects is usually calculated from Newton's laws of motion and law of universal gravitation. Orbital mechanics is a core discipline within space-mission design and control.

In organic chemistry, aromaticity is a property of cyclic (ring-shaped), planar (flat) structures with a ring of resonance bonds that gives increased stability compared to other geometric or connective arrangements with the same set of atoms. Aromatic molecules are very stable, and do not break apart easily to react with other substances. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have special stability.

The octet rule is a chemical rule of thumb that reflects the observation that elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens, but also to metals such as sodium or magnesium.
Hund's rule of maximum multiplicity is a rule based on observation of atomic spectra, which is used to predict the ground state of an atom or molecule with one or more open electronic shells. The rule states that for a given electron configuration, the lowest energy term is the one with the greatest value of spin multiplicity. This implies that if two or more orbitals of equal energy are available, electrons will occupy them singly before filling them in pairs. The rule, discovered by Friedrich Hund in 1925, is of important use in atomic chemistry, spectroscopy, and quantum chemistry, and is often abbreviated to Hund's rule, ignoring Hund's other two rules.

Quantum numbers describe values of conserved quantities in the dynamics of a quantum system. In the case of electrons, the quantum numbers can be defined as "the sets of numerical values which give acceptable solutions to the Schrödinger wave equation for the hydrogen atom". In more general cases, quantum numbers correspond to eigenvalues of operators that commute with the Hamiltonian—quantities that can be known with precision at the same time as the system's energy—and their corresponding eigenspaces. Together, a specification of all of the quantum numbers of a quantum system fully characterize a basis state of the system, and can in principle be measured together.

In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is symmetrical with respect to rotation about the bond axis. By this definition, common forms of sigma bonds are s+s, pz+pz, s+pz and dz2+dz2 . Quantum theory also indicates that molecular orbitals (MO) of identical symmetry actually mix or hybridize. As a practical consequence of this mixing of diatomic molecules, the wavefunctions s+s and pz+pz molecular orbitals become blended. The extent of this mixing depends on the relative energies of the MOs of like symmetry.
In chemistry, orbital hybridisation is the concept of mixing atomic orbitals into new hybrid orbitals suitable for the pairing of electrons to form chemical bonds in valence bond theory. Hybrid orbitals are very useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Although sometimes taught together with the valence shell electron-pair repulsion (VSEPR) theory, valence bond and hybridisation are in fact not related to the VSEPR model.

The aufbau principle, from the German Aufbauprinzip, also called the aufbau rule, states that in the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy levels before occupying higher levels. For example, the 1s subshell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable electron configuration possible. An example is the configuration 1s2 2s2 2p6 3s2 3p3 for the phosphorus atom, meaning that the 1s subshell has 2 electrons, and so on.
In atomic physics, Hund's rules refers to a set of rules that German physicist Friedrich Hund formulated around 1927, which are used to determine the term symbol that corresponds to the ground state of a multi-electron atom. The first rule is especially important in chemistry, where it is often referred to simply as Hund's Rule.
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such as borane and carborane clusters. The electron counting rules were originally formulated by Kenneth Wade and were further developed by Michael Mingos and others; they are sometimes known as Wade's rules or the Wade–Mingos rules. The rules are based on a molecular orbital treatment of the bonding. These rules have been extended and unified in the form of the Jemmis mno rules.
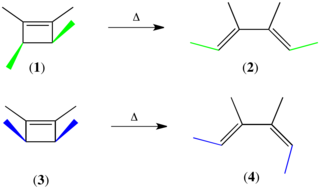
The Woodward–Hoffmann rules, devised by Robert Burns Woodward and Roald Hoffmann, are a set of rules used to rationalize or predict certain aspects of the stereochemistry and activation energy of pericyclic reactions, an important class of reactions in organic chemistry. The Woodward–Hoffmann rules are a consequence of the changes in electronic structure that occur during a pericyclic reaction and are predicated on the phasing of the interacting molecular orbitals. They are applicable to all classes of pericyclic reactions, including (1) electrocyclizations, (2) cycloadditions, (3) sigmatropic reactions, (4) group transfer reactions, (5) ene reactions, (6) cheletropic reactions, and (7) dyotropic reactions. Due to their elegance, simplicity, and generality, the Woodward–Hoffmann rules are credited with first exemplifying the power of molecular orbital theory to experimental chemists.
The 18-electron rule is a rule used primarily for predicting and rationalizing formulas for stable metal complexes, especially organometallic compounds. The rule is based on the fact that the valence shells of transition metals consist of nine valence orbitals, which collectively can accommodate 18 electrons as either bonding or nonbonding electron pairs. This means that the combination of these nine atomic orbitals with ligand orbitals creates nine molecular orbitals that are either metal-ligand bonding or non-bonding. When a metal complex has 18 valence electrons, it is said to have achieved the same electron configuration as the noble gas in the period. The rule and its exceptions are similar to the application of the octet rule to main group elements. The rule is not helpful for complexes of metals that are not transition metals, and interesting or useful transition metal complexes will violate the rule because of the consequences deviating from the rule bears on reactivity. The rule was first proposed by American chemist Irving Langmuir in 1921.
Naltor is a fictional planet first depicted in stories of DC Comics' Legion of Super-Heroes set in the 30th and 31st Centuries. It was portrayed as being the home of several Legion members, most prominently Dream Girl and her sister White Witch.

American football, referred to as football in the United States and Canada and also known as gridiron, is a team sport played by two teams of eleven players on a rectangular field with goalposts at each end. The offense, the team with possession of the oval-shaped football, attempts to advance down the field by running with the ball or passing it, while the defense, the team without possession of the ball, aims to stop the offense's advance and to take control of the ball for themselves. The offense must advance at least ten yards in four downs or plays; if they fail, they turn over the football to the defense, but if they succeed, they are given a new set of four downs to continue the drive. Points are scored primarily by advancing the ball into the opposing team's end zone for a touchdown or kicking the ball through the opponent's goalposts for a field goal. The team with the most points at the end of a game wins.

Sport includes all forms of competitive physical activity or games which, through casual or organised participation, at least in part aim to use, maintain or improve physical ability and skills while providing enjoyment to participants, and in some cases, entertainment for spectators. Hundreds of sports exist, from those between single contestants, through to those with hundreds of simultaneous participants, either in teams or competing as individuals. In certain sports such as racing, many contestants may compete, simultaneously or consecutively, with one winner; in others, the contest is between two sides, each attempting to exceed the other. Some sports allow a "tie" or "draw", in which there is no single winner; others provide tie-breaking methods to ensure one winner and one loser. A number of contests may be arranged in a tournament producing a champion. Many sports leagues make an annual champion by arranging games in a regular sports season, followed in some cases by playoffs.












