Related Research Articles

Acetylcholine (ACh) is an organic compound that functions in the brain and body of many types of animals as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic.

A motor neuron is a neuron whose cell body is located in the motor cortex, brainstem or the spinal cord, and whose axon (fiber) projects to the spinal cord or outside of the spinal cord to directly or indirectly control effector organs, mainly muscles and glands. There are two types of motor neuron – upper motor neurons and lower motor neurons. Axons from upper motor neurons synapse onto interneurons in the spinal cord and occasionally directly onto lower motor neurons. The axons from the lower motor neurons are efferent nerve fibers that carry signals from the spinal cord to the effectors. Types of lower motor neurons are alpha motor neurons, beta motor neurons, and gamma motor neurons.
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential. IPSPs were first investigated in motorneurons by David P. C. Lloyd, John Eccles and Rodolfo Llinás in the 1950s and 1960s. The opposite of an inhibitory postsynaptic potential is an excitatory postsynaptic potential (EPSP), which is a synaptic potential that makes a postsynaptic neuron more likely to generate an action potential. IPSPs can take place at all chemical synapses, which use the secretion of neurotransmitters to create cell to cell signalling. Inhibitory presynaptic neurons release neurotransmitters that then bind to the postsynaptic receptors; this induces a change in the permeability of the postsynaptic neuronal membrane to particular ions. An electric current that changes the postsynaptic membrane potential to create a more negative postsynaptic potential is generated, i.e. the postsynaptic membrane potential becomes more negative than the resting membrane potential, and this is called hyperpolarisation. To generate an action potential, the postsynaptic membrane must depolarize—the membrane potential must reach a voltage threshold more positive than the resting membrane potential. Therefore, hyperpolarisation of the postsynaptic membrane makes it less likely for depolarisation to sufficiently occur to generate an action potential in the postsynaptic neurone.
In neurophysiology, long-term depression (LTD) is an activity-dependent reduction in the efficacy of neuronal synapses lasting hours or longer following a long patterned stimulus. LTD occurs in many areas of the CNS with varying mechanisms depending upon brain region and developmental progress.
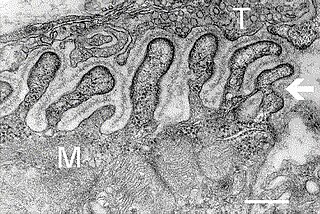
A neuromuscular junction is a chemical synapse between a motor neuron and a muscle fiber.

Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as when holding something heavy in the same position. The termination of muscle contraction is followed by muscle relaxation, which is a return of the muscle fibers to their low tension-generating state.
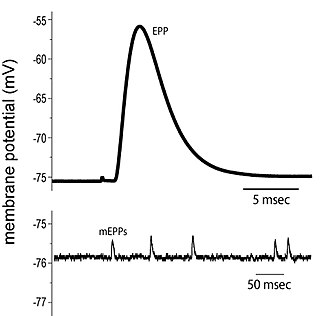
End plate potentials (EPPs) are the voltages which cause depolarization of skeletal muscle fibers caused by neurotransmitters binding to the postsynaptic membrane in the neuromuscular junction. They are called "end plates" because the postsynaptic terminals of muscle fibers have a large, saucer-like appearance. When an action potential reaches the axon terminal of a motor neuron, vesicles carrying neurotransmitters are exocytosed and the contents are released into the neuromuscular junction. These neurotransmitters bind to receptors on the postsynaptic membrane and lead to its depolarization. In the absence of an action potential, acetylcholine vesicles spontaneously leak into the neuromuscular junction and cause very small depolarizations in the postsynaptic membrane. This small response (~0.4mV) is called a miniature end plate potential (MEPP) and is generated by one acetylcholine-containing vesicle. It represents the smallest possible depolarization which can be induced in a muscle.
Synaptogenesis is the formation of synapses between neurons in the nervous system. Although it occurs throughout a healthy person's lifespan, an explosion of synapse formation occurs during early brain development, known as exuberant synaptogenesis. Synaptogenesis is particularly important during an individual's critical period, during which there is a certain degree of synaptic pruning due to competition for neural growth factors by neurons and synapses. Processes that are not used, or inhibited during their critical period will fail to develop normally later on in life.

A neural circuit is a population of neurons interconnected by synapses to carry out a specific function when activated. Multiple neural circuits interconnect with one another to form large scale brain networks.
Lower motor neurons (LMNs) are motor neurons located in either the anterior grey column, anterior nerve roots or the cranial nerve nuclei of the brainstem and cranial nerves with motor function. Many voluntary movements rely on spinal lower motor neurons, which innervate skeletal muscle fibers and act as a link between upper motor neurons and muscles. Cranial nerve lower motor neurons also control some voluntary movements of the eyes, face and tongue, and contribute to chewing, swallowing and vocalization. Damage to the lower motor neurons can lead to flaccid paralysis, absent deep tendon reflexes and muscle atrophy.

Motor unit recruitment is the activation of additional motor units to accomplish an increase in contractile strength in a muscle. A motor unit consists of one motor neuron and all of the muscle fibers it stimulates. All muscles consist of a number of motor units and the fibers belonging to a motor unit are dispersed and intermingle amongst fibers of other units. The muscle fibers belonging to one motor unit can be spread throughout part, or most of the entire muscle, depending on the number of fibers and size of the muscle. When a motor neuron is activated, all of the muscle fibers innervated by the motor neuron are stimulated and contract. The activation of one motor neuron will result in a weak but distributed muscle contraction. The activation of more motor neurons will result in more muscle fibers being activated, and therefore a stronger muscle contraction. Motor unit recruitment is a measure of how many motor neurons are activated in a particular muscle, and therefore is a measure of how many muscle fibers of that muscle are activated. The higher the recruitment the stronger the muscle contraction will be. Motor units are generally recruited in order of smallest to largest as contraction increases. This is known as Henneman's size principle.
Neural adaptation or sensory adaptation is a gradual decrease over time in the responsiveness of the sensory system to a constant stimulus. It is usually experienced as a change in the stimulus. For example, if a hand is rested on a table, the table's surface is immediately felt against the skin. Subsequently, however, the sensation of the table surface against the skin gradually diminishes until it is virtually unnoticeable. The sensory neurons that initially respond are no longer stimulated to respond; this is an example of neural adaptation.

Neural oscillations, or brainwaves, are rhythmic or repetitive patterns of neural activity in the central nervous system. Neural tissue can generate oscillatory activity in many ways, driven either by mechanisms within individual neurons or by interactions between neurons. In individual neurons, oscillations can appear either as oscillations in membrane potential or as rhythmic patterns of action potentials, which then produce oscillatory activation of post-synaptic neurons. At the level of neural ensembles, synchronized activity of large numbers of neurons can give rise to macroscopic oscillations, which can be observed in an electroencephalogram. Oscillatory activity in groups of neurons generally arises from feedback connections between the neurons that result in the synchronization of their firing patterns. The interaction between neurons can give rise to oscillations at a different frequency than the firing frequency of individual neurons. A well-known example of macroscopic neural oscillations is alpha activity.
The preBötzinger complex, often abbreviated as preBötC, is a functionally and anatomically specialized site in the ventral-lateral region of the lower medulla oblongata. The preBötC is part of the ventral respiratory group of respiratory related interneurons. Its foremost function is to generate the inspiratory breathing rhythm in mammals. In addition, the preBötC is widely and paucisynaptically connected to higher brain centers that regulate arousal and excitability more generally such that respiratory brain function is intimately connected with many other rhythmic and cognitive functions of the brain and central nervous system. Further, the preBötC receives mechanical sensory information from the airways that encode lung volume as well as pH, oxygen, and carbon dioxide content of circulating blood and the cerebrospinal fluid.

Nonsynaptic plasticity is a form of neuroplasticity that involves modification of ion channel function in the axon, dendrites, and cell body that results in specific changes in the integration of excitatory postsynaptic potentials and inhibitory postsynaptic potentials. Nonsynaptic plasticity is a modification of the intrinsic excitability of the neuron. It interacts with synaptic plasticity, but it is considered a separate entity from synaptic plasticity. Intrinsic modification of the electrical properties of neurons plays a role in many aspects of plasticity from homeostatic plasticity to learning and memory itself. Nonsynaptic plasticity affects synaptic integration, subthreshold propagation, spike generation, and other fundamental mechanisms of neurons at the cellular level. These individual neuronal alterations can result in changes in higher brain function, especially learning and memory. However, as an emerging field in neuroscience, much of the knowledge about nonsynaptic plasticity is uncertain and still requires further investigation to better define its role in brain function and behavior.
A motor pool consists of all individual motor neurons that innervate a single muscle. Each individual muscle fiber is innervated by only one motor neuron, but one motor neuron may innervate several muscle fibers. This distinction is physiologically significant because the size of a given motor pool determines the activity of the muscle it innervates: for example, muscles responsible for finer movements are innervated by motor pools consisting of higher numbers of individual motor neurons. Motor pools are also distinguished by the different classes of motor neurons that they contain. The size, composition, and anatomical location of each motor pool is tightly controlled by complex developmental pathways.
In neuroscience, synaptic scaling is a form of homeostatic plasticity, in which the brain responds to chronically elevated activity in a neural circuit with negative feedback, allowing individual neurons to reduce their overall action potential firing rate. Where Hebbian plasticity mechanisms modify neural synaptic connections selectively, synaptic scaling normalizes all neural synaptic connections by decreasing the strength of each synapse by the same factor, so that the relative synaptic weighting of each synapse is preserved.
Henneman’s size principle describes relationships between properties of motor neurons and the muscle fibers they innervate and thus control, which together are called motor units. Motor neurons with large cell bodies tend to innervate fast-twitch, high-force, less fatigue-resistant muscle fibers, whereas motor neurons with small cell bodies tend to innervate slow-twitch, low-force, fatigue-resistant muscle fibers. In order to contract a particular muscle, motor neurons with small cell bodies are recruited before motor neurons with large cell bodies. It was proposed by Elwood Henneman.
Perisynaptic schwann cells are neuroglia found at the Neuromuscular junction (NMJ) with known functions in synaptic transmission, synaptogenesis, and nerve regeneration. These cells share a common ancestor with both Myelinating and Non-Myelinating Schwann Cells called Neural Crest cells. Perisynaptic Schwann Cells (PSCs) contribute to the tripartite synapse organization in combination with the pre-synaptic nerve and the post-synaptic muscle fiber. PSCs are considered to be the glial component of the Neuromuscular Junction (NMJ) and have a similar functionality to that of Astrocytes in the Central Nervous System. The characteristics of PSCs are based on both external synaptic properties and internal glial properties, where the internal characteristics of PSCs develop based on the associated synapse, for example: the PSCs of a fast-twitch muscle fiber differ from the PSCs of a slow-twitch muscle fiber even when removed from their natural synaptic environment. PSCs of fast-twitch muscle fibers have higher Calcium levels in response to synapse innervation when compared to slow-twitch PSCs. This balance between external and internal influences creates a range of PSCs that are present in the many Neuromuscular Junctions of the Peripheral Nervous System.

Eberhard Erich Fetz is an American neuroscientist, academic and researcher. He is a Professor of Physiology and Biophysics and DXARTS at the University of Washington.
References
- ↑ Physical Activity: Strength Training for Older Adults. Center for Disease Control and Prevention. (2010).
- 1 2 3 Aagaard, P. (2003). Training-induced changes in neuronal function. Exercise and Sport Sciences Reviews. 31(2). 61-67.
- ↑ Carroll, T. J., Riek, S., & Carson, R. G. (2001). Neural adaptations to resistance training - Implications for movement control. Sports Medicine, 31(12), 829–840.
- ↑ Duchateau, J., Semmler, J. G., & Enoka, R. M. (2006). Training adaptations in the behavior of human motor units. Journal of Applied Physiology, 101(6), 1766-1775. doi : 10.1152/japplphysiol.00543.2006
- 1 2 Bawa, P. (2002). Neural control of motor output: Can training change it? Exercise and Sport Sciences Reviews, 30(2), 59-63.
- ↑ Klass, M., Baudry, S., & Duchateau, J. (2008). Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. Journal of Applied Physiology, 10, 739-746.
- ↑ Deschenes, M. R., & Wilson, M. H. (2003). Age-related differences in synaptic plasticity following muscle unloading. Journal of Neurobiology, 57(3), 246-256. doi : 10.1002/neu.10271
- ↑ Clamann, H. P. (1993). MOTOR UNIT RECRUITMENT AND THE GRADATION OF MUSCLE FORCE. Physical Therapy, 73(12), 830-843.
- ↑ Bakels, R., & Kernell, D. (1993). MATCHING BETWEEN MOTONEURON AND MUSCLE UNIT PROPERTIES IN RAT MEDIAL GASTROCNEMIUS. Journal of Physiology-London, 463, 307-324.
- ↑ Deschenes, M.R., Tenny, K.A., & Wilson, M.H. (2006). Increased and decreased activity elicits specific morphological adaptations of the neuromuscular junction. Neuroscience, 137(4):1277-83.
- ↑ Markovic, G. & Mikulic, P. (2010) Neuro-musculoskeletal and performance adaptations to lower-extremity plyometric training. Sports Med, 40(10):859-95. doi : 10.2165/11318370
- ↑ Vanderheyden, M. J., Hilgevoord, A. A. J., Bour, L. J., & Devisser, B. W. O. (1994). MODELING MOTONEURON FIRING PROPERTIES - DEPENDENCY ON SIZE AND CALCIUM DYNAMICS. Biological Cybernetics, 72(2), 133-139