Related Research Articles
Transcatheter arterial chemoembolization (TACE) is a minimally invasive procedure performed in interventional radiology to restrict a tumor's blood supply. Small embolic particles coated with chemotherapeutic drugs are injected selectively through a catheter into an artery directly supplying the tumor. These particles both block the blood supply and induce cytotoxicity, attacking the tumor in several ways.

Azacitidine, sold under the brand name Vidaza among others, is a medication used for the treatment of myelodysplastic syndrome, myeloid leukemia, and juvenile myelomonocytic leukemia. It is a chemical analog of cytidine, a nucleoside in DNA and RNA. Azacitidine and its deoxy derivative, decitabine were first synthesized in Czechoslovakia as potential chemotherapeutic agents for cancer.

Thiopurine methyltransferase or thiopurine S-methyltransferase (TPMT) is an enzyme that in humans is encoded by the TPMT gene. A pseudogene for this locus is located on chromosome 18q.

Streptozotocin or streptozocin (STZ) is a naturally occurring alkylating antineoplastic agent that is particularly toxic to the insulin-producing beta cells of the pancreas in mammals. It is used in medicine for treating certain cancers of the islets of Langerhans and used in medical research to produce an animal model for hyperglycemia and Alzheimer's in a large dose, as well as type 2 diabetes or type 1 diabetes with multiple low doses.

KRAS is a gene that provides instructions for making a protein called K-Ras, a part of the RAS/MAPK pathway. The protein relays signals from outside the cell to the cell's nucleus. These signals instruct the cell to grow and divide (proliferate) or to mature and take on specialized functions (differentiate). It is called KRAS because it was first identified as a viral oncogene in the KirstenRAt Sarcoma virus. The oncogene identified was derived from a cellular genome, so KRAS, when found in a cellular genome, is called a proto-oncogene.
A humanized mouse is a genetically modified mouse that has functioning human genes, cells, tissues and/or organs. Humanized mice are commonly used as small animal models in biological and medical research for human therapeutics.
The NSG mouse is a brand of immunodeficient laboratory mice, developed and marketed by Jackson Laboratory, which carries the strain NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ. NSG branded mice are among the most immunodeficient described to date. NSG branded mice lack mature T cells, B cells, and natural killer (NK) cells. NSG branded mice are also deficient in multiple cytokine signaling pathways, and they have many defects in innate immunity. The compound immunodeficiencies in NSG branded mice permit the engraftment of a wide range of primary human cells, and enable sophisticated modeling of many areas of human biology and disease. NSG branded mice were developed in the laboratory of Dr. Leonard Shultz at Jackson Laboratory, which owns the NSG trade mark.
Pelareorep is a proprietary isolate of the unmodified human reovirus being developed as a systemically administered immuno-oncological viral agent for the treatment of solid tumors and hematological malignancies. Pelareorep is an oncolytic virus, which means that it preferentially lyses cancer cells. Pelareorep also promotes an inflamed tumor phenotype through innate and adaptive immune responses. Preliminary clinical trials indicate that it may have anti-cancer effects across a variety of cancer types when administered alone and in combination with other cancer therapies.

Cell encapsulation is a possible solution to graft rejection in tissue engineering applications. Cell microencapsulation technology involves immobilization of cells within a polymeric semi-permeable membrane. It permits the bidirectional diffusion of molecules such as the influx of oxygen, nutrients, growth factors etc. essential for cell metabolism and the outward diffusion of waste products and therapeutic proteins. At the same time, the semi-permeable nature of the membrane prevents immune cells and antibodies from destroying the encapsulated cells, regarding them as foreign invaders.
Lewis lung carcinoma is a hypermutated Kras/Nras–mutant cancer with extensive regional mutation clusters in its genome. A tumor that spontaneously developed as an epidermoid carcinoma in the lung of a C57BL mouse. It was discovered in 1951 by Dr. Margaret Lewis of the Wistar Institute and became one of the first transplantable tumors.

The abscopal effect is a hypothesis in the treatment of metastatic cancer whereby shrinkage of untreated tumors occurs concurrently with shrinkage of tumors within the scope of the localized treatment. R.H. Mole proposed the term “abscopal” in 1953 to refer to effects of ionizing radiation “at a distance from the irradiated volume but within the same organism.”

Sonodynamic therapy (SDT) is a noninvasive treatment, often used for tumor irradiation, that utilizes a sonosensitizer and the deep penetration of ultrasound to treat lesions of varying depths by reducing target cell number and preventing future tumor growth. Many existing cancer treatment strategies cause systemic toxicity or cannot penetrate tissue deep enough to reach the entire tumor; however, emerging ultrasound stimulated therapies could offer an alternative to these treatments with their increased efficiency, greater penetration depth, and reduced side effects. Sonodynamic therapy could be used to treat cancers and other diseases, such as atherosclerosis, and diminish the risk associated with other treatment strategies since it induces cytotoxic effects only when externally stimulated by ultrasound and only at the cancerous region, as opposed to the systemic administration of chemotherapy drugs.
Micro-Culture Kinetic (MiCK) assay, developed by DiaTech Oncology, is a clinical pathology test that measures apoptosis induced in specific patient's cancer cells via chemotherapy. The assay is performed by DiaTech Oncology in its CLAI-certified, CAP-accredited laboratory. The MiCK assay provides oncologists with a clinically relevant drug-sensitivity profile of tumor cells within an individual cancer patient.
Targeted molecular therapy for neuroblastoma involves treatment aimed at molecular targets that have a unique expression in this form of cancer. Neuroblastoma, the second most common pediatric malignant tumor, often involves treatment through intensive chemotherapy. A number of molecular targets have been identified for the treatment of high-risk forms of this disease. Aiming treatment in this way provides a more selective way to treat the disease, decreasing the risk for toxicities that are associated with the typical treatment regimen. Treatment using these targets can supplement or replace some of the intensive chemotherapy that is used for neuroblastoma. These molecular targets of this disease include GD2, ALK, and CD133. GD2 is a target of immunotherapy, and is the most fully developed of these treatment methods, but is also associated with toxicities. ALK has more recently been discovered, and drugs in development for this target are proving to be successful in neuroblastoma treatment. The role of CD133 in neuroblastoma has also been more recently discovered and is an effective target for treatment of this disease.
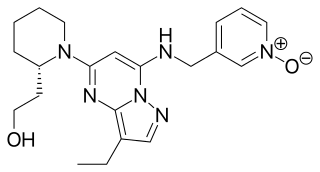
Dinaciclib (SCH-727965) is an experimental drug that inhibits cyclin-dependent kinases (CDKs). It is being evaluated in clinical trials for various cancer indications.

Champions Oncology is an American technology company that develops mouse avatars. Called TumorGrafts, they are used to test a panel of chemotherapy regimens, targeted therapies and monoclonal antibodies to identify potential therapeutic options for cancer patients. The company was founded in 2007 by David Sidransky, M.D., a Johns Hopkins University oncologist.
Patient derived xenografts (PDX) are models of cancer where the tissue or cells from a patient's tumor are implanted into an immunodeficient or humanized mouse. It is a form of xenotransplantation. PDX models are used to create an environment that allows for the continued growth of cancer after its removal from a patient. In this way, tumor growth can be monitored in the laboratory, including in response to potential therapeutic options. Cohorts of PDX models can be used to determine the therapeutic efficiency of a therapy against particular types of cancer, or a PDX model from a specific patient can be tested against a range of therapies in a 'personalized oncology' approach.
Manuel Hidalgo Medina is an oncologist. He is director of the Leon V. and Marilyn L. Rosenberg Clinical Cancer Center at Beth Israel Deaconess Medical Center in Boston, Massachusetts, in the United States; he also heads its hematology/oncology division. He specializes in pancreatic cancer. His research has included development of anti-cancer drugs such as erlotinib, nab-paclitaxel and temsirolimus, and the development of patient-derived tumor xenograft models.
Adeno-associated virus (AAV) has been researched as a viral vector in gene therapy for cancer treatment as an oncolytic virus. Currently there are not any FDA approved AAV cancer treatments, as the first FDA approved AAV treatment was approved December 2017. However, there are many Oncolytic AAV applications that are in development and have been researched.

The laboratory mouse has been instrumental in investigating the genetics of human disease, including cancer, for over 110 years. The laboratory mouse has physiology and genetic characteristics very similar to humans providing powerful models for investigation of the genetic characteristics of disease.
References
- 1 2 Hidalgo M, Bruckheimer E, Rajeshkumar NV, et al: A pilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol Cancer Ther 10:1311-6, 2011
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2012-02-03. Retrieved 2012-04-13.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Dennis, Carina (2012). "Mouse 'avatars' could aid pancreatic cancer therapy". Nature. doi:10.1038/nature.2012.10259. S2CID 71515017.
- ↑ "Applying Bioinformatics to Precision Medicine".
- ↑ "Mayo Clinic".
- ↑ Pollack, Andrew (26 September 2012). "Seeking Cures, Patients Enlist Mice Stand-Ins". The New York Times.
- ↑ "Mouse Avatars Offer Hope for Personalized Cancer Treatment | New York Genome Center". Archived from the original on 2013-04-15. Retrieved 2013-03-06.
- ↑ "Of Mice and Man | Science & Society | Science News". Archived from the original on 2013-03-11. Retrieved 2013-03-13.
- ↑ Rubio-Viqueira B, Hidalgo M: Direct in vivo xenograft tumor model for predicting chemotherapeutic drug response in cancer patients. Clin Pharmacol Ther 85:217-21, 2009
- ↑ Garrido-Laguna I, Uson M, Rajeshkumar NV, et al: Tumor engraftment in nude mice and enrichment in stroma-related gene pathways predicts poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin Cancer Res, 2011
- ↑ Morelli MP, Calvo E, Ordonez E, et al: Prioritizing Phase I Treatment Options Through Preclinical Testing on Personalized Tumorgraft. J Clin Oncol, 2011
- ↑ Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, et al: Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol Cancer Ther 10:3-8, 2011